Heme Mobilization in Animals: A Metallolipid's Journey
- PMID: 27254265
- PMCID: PMC5629413
- DOI: 10.1021/acs.accounts.5b00553
Heme Mobilization in Animals: A Metallolipid's Journey
Abstract
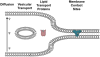
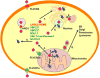
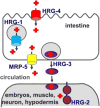
Heme is universally recognized as an essential and ubiquitous prosthetic group that enables proteins to carry out a diverse array of functions. All heme-dependent processes, from protein hemylation to heme signaling, require the dynamic and rapid mobilization of heme to hemoproteins present in virtually every subcellular compartment. The cytotoxicity and hydrophobicity of heme necessitates that heme mobilization is carefully controlled at the cellular and systemic level. However, the molecules and mechanisms that mediate heme homeostasis are poorly understood. In this Account, we provide a heuristic paradigm with which to conceptualize heme trafficking and highlight the most recent developments in the mechanisms underlying heme trafficking. As an iron-containing tetrapyrrole, heme exhibits properties of both transition metals and lipids. Accordingly, we propose its transport and trafficking will reflect principles gleaned from the trafficking of both metals and lipids. Using this conceptual framework, we follow the flow of heme from the final step of heme synthesis in the mitochondria to hemoproteins present in various subcellular organelles. Further, given that many cells and animals that cannot make heme can assimilate it intact from nutritional sources, we propose that intercellular heme trafficking pathways must exist. This necessitates that heme be able to be imported and exported from cells, escorted between cells and organs, and regulated at the organismal level via a coordinated systemic process. In this Account, we highlight recently discovered heme transport and trafficking factors and provide the biochemical foundation for the cell and systems biology of heme. Altogether, we seek to reconceptualize heme from an exchange inert cofactor buried in hemoprotein active sites to an exchange labile and mobile metallonutrient.
Figures

References
-
- Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, Li D, Wu Y, Shang Y, Kong X, Yu L, Li L, Ruan K, Hu H, Huang Y, Hui L, Xie D, Wang F, Hu R. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014;7:180–193. - PMC - PubMed
-
- Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

