NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies
- PMID: 27254664
- PMCID: PMC4890852
- DOI: 10.1371/journal.pbio.1002472
NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies
Abstract
Nicotinamide mononucleotide adenylyl transferase 2 (NMNAT2) is neuroprotective in numerous preclinical models of neurodegeneration. Here, we show that brain nmnat2 mRNA levels correlate positively with global cognitive function and negatively with AD pathology. In AD brains, NMNAT2 mRNA and protein levels are reduced. NMNAT2 shifts its solubility and colocalizes with aggregated Tau in AD brains, similar to chaperones, which aid in the clearance or refolding of misfolded proteins. Investigating the mechanism of this observation, we discover a novel chaperone function of NMNAT2, independent from its enzymatic activity. NMNAT2 complexes with heat shock protein 90 (HSP90) to refold aggregated protein substrates. NMNAT2's refoldase activity requires a unique C-terminal ATP site, activated in the presence of HSP90. Furthermore, deleting NMNAT2 function increases the vulnerability of cortical neurons to proteotoxic stress and excitotoxicity. Interestingly, NMNAT2 acts as a chaperone to reduce proteotoxic stress, while its enzymatic activity protects neurons from excitotoxicity. Taken together, our data indicate that NMNAT2 exerts its chaperone or enzymatic function in a context-dependent manner to maintain neuronal health.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

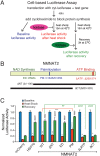
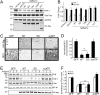
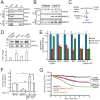

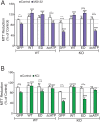
Comment in
-
Two for the Price of One: A Neuroprotective Chaperone Kit within NAD Synthase Protein NMNAT2.PLoS Biol. 2016 Jul 25;14(7):e1002522. doi: 10.1371/journal.pbio.1002522. eCollection 2016 Jul. PLoS Biol. 2016. PMID: 27454736 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- RF1 AG015819/AG/NIA NIH HHS/United States
- R01 NS086794/NS/NINDS NIH HHS/United States
- K08 AG034290/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- R01 NS048884/NS/NINDS NIH HHS/United States
- R01 AG023173/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- R01 AG042890/AG/NIA NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- T32 NS043124/NS/NINDS NIH HHS/United States
- R01 AG011101/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- R01 AG030146/AG/NIA NIH HHS/United States
- U24 NS051872/NS/NINDS NIH HHS/United States
- K01 AG024079/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- R01 NS059873/NS/NINDS NIH HHS/United States
- R01 AG023193/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

