Desmoglein 3 regulates membrane trafficking of cadherins, an implication in cell-cell adhesion
- PMID: 27254775
- PMCID: PMC5479455
- DOI: 10.1080/19336918.2016.1195942
Desmoglein 3 regulates membrane trafficking of cadherins, an implication in cell-cell adhesion
Abstract
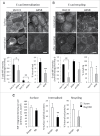
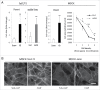
E-cadherin mediated cell-cell adhesion plays a critical role in epithelial cell polarization and morphogenesis. Our recent studies suggest that the desmosomal cadherin, desmoglein 3 (Dsg3) cross talks with E-cadherin and regulates its adhesive function in differentiating keratinocytes. However, the underlying mechanism remains not fully elucidated. Since E-cadherin trafficking has been recognized to be a central determinant in cell-cell adhesion and homeostasis we hypothesize that Dsg3 may play a role in regulating E-cadherin trafficking and hence the cell-cell adhesion. Here we investigated this hypothesis in cells with loss of Dsg3 function through RNAi mediated Dsg3 knockdown or the stable expression of the truncated mutant Dsg3ΔC. Our results showed that loss of Dsg3 resulted in compromised cell-cell adhesion and reduction of adherens junction and desmosome protein expression as well as the cortical F-actin formation. As a consequence, cells failed to polarize but instead displayed aberrant cell flattening. Furthermore, retardation of E-cadherin internalization and recycling was consistently observed in these cells during the process of calcium induced junction assembling. In contrast, enhanced cadherin endocytosis was detected in cells with overexpression of Dsg3 compared to control cells. Importantly, this altered cadherin trafficking was found to be coincided with the reduced expression and activity of Rab proteins, including Rab5, Rab7 and Rab11 which are known to be involved in E-cadherin trafficking. Taken together, our findings suggest that Dsg3 functions as a key in cell-cell adhesion through at least a mechanism of regulating E-cadherin membrane trafficking.
Keywords: E-cadherin trafficking; adherens junction; desmoglein 3; desmosome; epithelial cells; intercellular junction.
Figures








References
-
- Hennings H, Holbrook KA. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp. Cell Res 1983; 143 (1):127-42; PMID:6186504; https://doi.org/10.1016/0014-4827(83)90115-5 - DOI - PubMed
-
- Watt FM, Mattey DL, Garrod DR. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol 1984; 99 (6):2211-5; PMID:6209289; https://doi.org/10.1083/jcb.99.6.2211 - DOI - PMC - PubMed
-
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta 2008; 1778 (3):572-87; PMID:17854763; https://doi.org/10.1016/j.bbamem.2007.07.014 - DOI - PubMed
-
- Kanno M, Isa Y, Aoyama Y, Yamamoto Y, Nagai M, Ozawa M, Kitajima Y. P120-catenin is a novel desmoglein 3 interacting partner: identification of the p120-catenin association site of desmoglein 3. Exp Cell Res 2008; 314(8):1683-92; PMID:18343367; https://doi.org/10.1016/j.yexcr.2008.01.031 - DOI - PubMed
-
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol 2012; 199 (2):365-80; PMID:23071156; https://doi.org/10.1083/jcb.201205029 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
