Hierarchical Targeting Strategy for Enhanced Tumor Tissue Accumulation/Retention and Cellular Internalization
- PMID: 27255214
- PMCID: PMC5014563
- DOI: 10.1002/adma.201601498
Hierarchical Targeting Strategy for Enhanced Tumor Tissue Accumulation/Retention and Cellular Internalization
Abstract
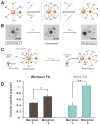
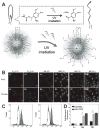
Targeted delivery of therapeutic agents is an important way to improve the therapeutic index and reduce side effects. To design nanoparticles for targeted delivery, both enhanced tumor tissue accumulation/retention and enhanced cellular internalization should be considered simultaneously. So far, there have been very few nanoparticles with immutable structures that can achieve this goal efficiently. Hierarchical targeting, a novel targeting strategy based on stimuli responsiveness, shows good potential to enhance both tumor tissue accumulation/retention and cellular internalization. Here, the recent design and development of hierarchical targeting nanoplatforms, based on changeable particle sizes, switchable surface charges and activatable surface ligands, will be introduced. In general, the targeting moieties in these nanoplatforms are not activated during blood circulation for efficient tumor tissue accumulation, but re-activated by certain internal or external stimuli in the tumor microenvironment for enhanced cellular internalization.
Keywords: activatable surface ligands; changeable particle sizes; hierarchical targeting; stimuli-responsive nanoplatforms; switchable surface charges.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



















References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

