Mechanisms, pools, and sites of spontaneous vesicle release at synapses of rod and cone photoreceptors
- PMID: 27255664
- PMCID: PMC4969212
- DOI: 10.1111/ejn.13288
Mechanisms, pools, and sites of spontaneous vesicle release at synapses of rod and cone photoreceptors
Abstract
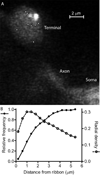
Photoreceptors have depolarized resting potentials that stimulate calcium-dependent release continuously from a large vesicle pool but neurons can also release vesicles without stimulation. We characterized the Ca(2+) dependence, vesicle pools, and release sites involved in spontaneous release at photoreceptor ribbon synapses. In whole-cell recordings from light-adapted horizontal cells (HCs) of tiger salamander retina, we detected miniature excitatory post-synaptic currents (mEPSCs) when no stimulation was applied to promote exocytosis. Blocking Ca(2+) influx by lowering extracellular Ca(2+) , by application of Cd(2+) and other agents reduced the frequency of mEPSCs but did not eliminate them, indicating that mEPSCs can occur independently of Ca(2+) . We also measured release presynaptically from rods and cones by examining quantal glutamate transporter anion currents. Presynaptic quantal event frequency was reduced by Cd(2+) or by increased intracellular Ca(2+) buffering in rods, but not in cones, that were voltage clamped at -70 mV. By inhibiting the vesicle cycle with bafilomycin, we found the frequency of mEPSCs declined more rapidly than the amplitude of evoked excitatory post-synaptic currents (EPSCs) suggesting a possible separation between vesicle pools in evoked and spontaneous exocytosis. We mapped sites of Ca(2+) -independent release using total internal reflectance fluorescence (TIRF) microscopy to visualize fusion of individual vesicles loaded with dextran-conjugated pHrodo. Spontaneous release in rods occurred more frequently at non-ribbon sites than evoked release events. The function of Ca(2+) -independent spontaneous release at continuously active photoreceptor synapses remains unclear, but the low frequency of spontaneous quanta limits their impact on noise.
Keywords: calcium; exocytosis; retina; ribbon synapse; spontaneous synaptic release; tiger salamander.
© 2016 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures





References
-
- Arancillo M, Min SW, Gerber S, Münster-Wandowski A, Wu YJ, Herman M, Trimbuch T, Rah JC, Ahnert-Hilger G, Riedel D, Südhof TC, Rosenmund C. Titration of Syntaxin1 in mammalian synapses reveals multiple roles in vesicle docking, priming, and release probability. The Journal of Neuroscience. 2013;33(42):16698–16714. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

