The bactericidal activity of glutaraldehyde-impregnated polyurethane
- PMID: 27255793
- PMCID: PMC5061724
- DOI: 10.1002/mbo3.378
The bactericidal activity of glutaraldehyde-impregnated polyurethane
Abstract
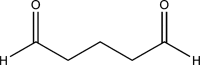
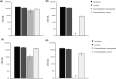
Although glutaraldehyde is known to be bactericidal in solution, its potential use to create novel antibacterial polymers suitable for use in healthcare environments has not been evaluated. Here, novel materials were prepared in which glutaraldehyde was either incorporated into polyurethane using a simple "swell-encapsulation-shrink" method (hereafter referred to as "glutaraldehyde-impregnated polyurethane"), or simply applied to the polymer surface (hereafter referred to as "glutaraldehyde-coated polyurethane"). The antibacterial activity of glutaraldehyde-impregnated and glutaraldehyde-coated polyurethane samples was tested against Escherichia coli and Staphylococcus aureus. Glutaraldehyde-impregnated polyurethane resulted in a 99.9% reduction in the numbers of E. coli within 2 h and a similar reduction of S. aureus within 1 h, whereas only a minimal reduction in bacterial numbers was observed when the biocide was bound to the polymer surface. After 15 days, however, the bactericidal activity of the impregnated material was substantially reduced presumably due to polymerization of glutaraldehyde. Thus, although glutaraldehyde retains antibacterial activity when impregnated into polyurethane, activity is not maintained for extended periods of time. Future work should examine the potential of chemical modification of glutaraldehyde and/or polyurethane to improve the useful lifespan of this novel antibacterial polymer.
Keywords: Antibacterial; biocide; glutaraldehyde; hospital-acquired infections; polymer.
© 2016 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures
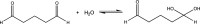
References
-
- Aiello, A. E. , Larson E. L., and Levy S. B.. 2007. Consumer antibacterial soaps: effective or just risky? Clin. Infect. Dis. 45:S137–S147. - PubMed
-
- Anon. 2003. Glutaraldehyde: Safe handling and storage guide. DOW Chemical Company.
-
- Blass, C. R . 1999. P. 58–60 Polymers in disposable medical devices: A European Perspective. iSmithers Rapra Publishing.
-
- D'Ercole, S. , Catamo G., De Fazio P., and Piccolomini R.. 2002. In vitro antimicrobial activity of glutaraldehyde plus O‐phenylphenol association. Minerva Stomatol. 51:29–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

