Resolvin D2 decreases TLR4 expression to mediate resolution in human monocytes
- PMID: 27256622
- PMCID: PMC5001508
- DOI: 10.1096/fj.201600375R
Resolvin D2 decreases TLR4 expression to mediate resolution in human monocytes
Abstract
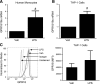
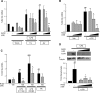
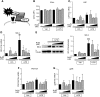
TLRs are critical for innate immunity, but excessive activation can lead to tissue damage and disease. Specialized proresolving mediators (SPMs), including resolvin D2 (RvD2), promote the active resolution of inflammation. How SPMs regulate early LPS signaling, including activation of TLR4, is unknown. We treated human THP-1 monocytic cells and primary human blood monocytes with RvD2 and LPS to evaluate modulation of TLRs. miRNA-146a overexpression and inhibition were used to dissect the mechanism of RvD2-mediated actions. We validated our studies using ELISAs for cytokines, PCR, Western blot analysis, and flow cytometry. Cells treated with 0.1% ethanol (control for RvD2) and/or PBS (control for LPS), and control microRNA mimics and inhibitors were used as controls. RvD2 reduced LPS-induced cytokines and TLR4 expression in human monocytes by up to 75%. In THP-1 cells, RvD2 reduced expression of TLR4, lymphocyte antigen 96 (MD-2), and downstream signals (MyD88, TRIF, and TAK1). These effects were partially mediated through RvD2 induction of microRNA-146a, and RvD2's actions were blocked by microRNA-146a inhibition. These new findings reveal the ability of RvD2 to reduce TLR4 expression and attenuate LPS-induced inflammation, providing a new area of SPM activity to investigate in this major area of therapeutic research.-Croasdell, A., Sime, P. J., Phipps, R. P. Resolvin D2 decreases TLR4 expression to mediate resolution in human monocytes.
Keywords: LPS; MD-2; SPM; inflammation; microRNA-146a.
© FASEB.
Figures





References
-
- Maris N. A., Dessing M. C., de Vos A. F., Bresser P., van der Zee J. S., Jansen H. M., Spek C. A., van der Poll T. (2006) Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur. Respir. J. 28, 622–626 - PubMed
-
- Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3, 667–672 - PubMed
-
- Szatmary Z. (2012) Molecular biology of Toll-like receptors. Gen. Physiol. Biophys. 31, 357–366 - PubMed
-
- Hall M. J., Williams S. N., DeFrances C. J., Golosinskiy A. (2011) Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 1–8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

