Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study
- PMID: 27256670
- PMCID: PMC5828512
- DOI: 10.1161/STROKEAHA.116.012995
Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study
Abstract
Background and purpose: Preclinical data suggest that cell-based therapies have the potential to improve stroke outcomes.
Methods: Eighteen patients with stable, chronic stroke were enrolled in a 2-year, open-label, single-arm study to evaluate the safety and clinical outcomes of surgical transplantation of modified bone marrow-derived mesenchymal stem cells (SB623).
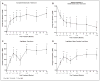
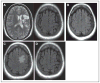
Results: All patients in the safety population (N=18) experienced at least 1 treatment-emergent adverse event. Six patients experienced 6 serious treatment-emergent adverse events; 2 were probably or definitely related to surgical procedure; none were related to cell treatment. All serious treatment-emergent adverse events resolved without sequelae. There were no dose-limiting toxicities or deaths. Sixteen patients completed 12 months of follow-up at the time of this analysis. Significant improvement from baseline (mean) was reported for: (1) European Stroke Scale: mean increase 6.88 (95% confidence interval, 3.5-10.3; P<0.001), (2) National Institutes of Health Stroke Scale: mean decrease 2.00 (95% confidence interval, -2.7 to -1.3; P<0.001), (3) Fugl-Meyer total score: mean increase 19.20 (95% confidence interval, 11.4-27.0; P<0.001), and (4) Fugl-Meyer motor function total score: mean increase 11.40 (95% confidence interval, 4.6-18.2; P<0.001). No changes were observed in modified Rankin Scale. The area of magnetic resonance T2 fluid-attenuated inversion recovery signal change in the ipsilateral cortex 1 week after implantation significantly correlated with clinical improvement at 12 months (P<0.001 for European Stroke Scale).
Conclusions: In this interim report, SB623 cells were safe and associated with improvement in clinical outcome end points at 12 months.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT01287936.
Keywords: Notch 1; allogeneic transplantation; mesenchymal stromal cells; phase 1 clinical trial; stem cells; stereotactic techniques; stroke.
© 2016 American Heart Association, Inc.
Figures
Comment in
-
Preliminary Reports of Stereotaxic Stem Cell Transplants in Chronic Stroke Patients.Mol Ther. 2016 Oct;24(10):1710-1711. doi: 10.1038/mt.2016.186. Mol Ther. 2016. PMID: 27818493 Free PMC article. No abstract available.
-
Neuroregeneration: North America's First Human Stem Cell Trial for Stroke.Neurosurgery. 2016 Dec;79(6):N21-N22. doi: 10.1227/01.neu.0000508607.84436.6d. Neurosurgery. 2016. PMID: 27861413 No abstract available.
-
Neuroscience: New nerves for old.Nature. 2016 Dec 7;540(7632):S52-S54. doi: 10.1038/540S52a. Nature. 2016. PMID: 27926699 No abstract available.
References
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. - DOI - PMC - PubMed
-
- George P, Steinberg GK. Cell based therapies for stroke. In: Micieli G, Amantea D, editors. Rational Basis for Clinical Translation in Stroke Therapy. Boca Raton, FL: CRC Press; 2014. pp. 427–445. (Frontiers in Neurotherapeutics Series)
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

