Tenascin-C drives persistence of organ fibrosis
- PMID: 27256716
- PMCID: PMC4895803
- DOI: 10.1038/ncomms11703
Tenascin-C drives persistence of organ fibrosis
Abstract
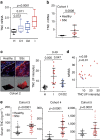
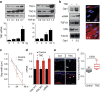
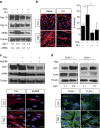
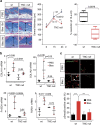
The factors responsible for maintaining persistent organ fibrosis in systemic sclerosis (SSc) are not known but emerging evidence implicates toll-like receptors (TLRs) in the pathogenesis of SSc. Here we show the expression, mechanism of action and pathogenic role of endogenous TLR activators in skin from patients with SSc, skin fibroblasts, and in mouse models of organ fibrosis. Levels of tenascin-C are elevated in SSc skin biopsy samples, and serum and SSc fibroblasts, and in fibrotic skin tissues from mice. Exogenous tenascin-C stimulates collagen gene expression and myofibroblast transformation via TLR4 signalling. Mice lacking tenascin-C show attenuation of skin and lung fibrosis, and accelerated fibrosis resolution. These results identify tenascin-C as an endogenous danger signal that is upregulated in SSc and drives TLR4-dependent fibroblast activation, and by its persistence impedes fibrosis resolution. Disrupting this fibrosis amplification loop might be a viable strategy for the treatment of SSc.
Figures

Comment in
-
Systemic sclerosis: Tenascin C perpetuates tissue fibrosis.Nat Rev Rheumatol. 2016 Jul;12(7):375. doi: 10.1038/nrrheum.2016.99. Epub 2016 Jun 9. Nat Rev Rheumatol. 2016. PMID: 27278125 No abstract available.
References
-
- Rockey D. C., Bell P. D. & Hill J. A. Fibrosis—a common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

