Patient Counseling and Management of Symptoms During Olaparib Therapy for Recurrent Ovarian Cancer
- PMID: 27256873
- PMCID: PMC4978548
- DOI: 10.1634/theoncologist.2015-0268
Patient Counseling and Management of Symptoms During Olaparib Therapy for Recurrent Ovarian Cancer
Abstract
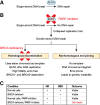
: Our primary objective is to review the safety and tolerability profile of olaparib, a novel anticancer therapy, and to discuss key considerations for symptom management in patients with advanced ovarian cancer. Olaparib is the first of a new class of anticancer therapies, poly (ADP-ribose) polymerase (PARP) inhibitors that target tumors that have deficits in homologous recombination repair (such as BRCA mutations) by a process known as synthetic lethality. Through this process, neither the deficiency in homologous recombination repair nor PARP inhibition alone is cytotoxic, but the combination of these two conditions leads to cell death. In December 2014, olaparib received accelerated approval by the U.S. Food and Drug Administration (FDA) as monotherapy for patients with known or suspected deleterious germline BRCA-mutated (as detected by an FDA-approved test) advanced ovarian cancer who had been treated with at least three lines of chemotherapy. Most adverse events (AEs) reported during olaparib clinical trials conducted in patients with recurrent ovarian cancer and measurable disease were of grade 2 or less severity according to the National Cancer Institute's Common Terminology Criteria for Adverse Events. Fatigue and gastrointestinal AEs are among the most common in ovarian cancer clinical trials and can be particularly bothersome to patients. We focus on interventions to address these AEs in patients who are candidates for treatment with olaparib and allow them to remain on therapy for as long as clinically indicated.
Implications for practice: Olaparib therapy represents a new approach to treating recurrent ovarian cancer. Some associated adverse events can have a substantial effect on quality of life. It is therefore important for patients, caregivers, and health care providers to have realistic expectations and a thorough understanding of the safety and tolerability profile of olaparib to prevent or alleviate key symptoms so that therapy can continue uninterrupted if possible. This report summarizes a practical approach to supportive care for patients receiving olaparib therapy.
Keywords: BRCA mutation; Homologous recombination pathway; Olaparib; Ovarian cancer; Poly(ADP-ribose) polymerase inhibitor (PARP); Toxicities.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
Clinical Application of Poly(ADP-Ribose) Polymerase Inhibitors in High-Grade Serous Ovarian Cancer.Oncologist. 2016 May;21(5):586-93. doi: 10.1634/theoncologist.2015-0438. Epub 2016 Mar 28. Oncologist. 2016. PMID: 27022037 Free PMC article. Review.
-
Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial.Lancet Oncol. 2015 Jan;16(1):87-97. doi: 10.1016/S1470-2045(14)71135-0. Epub 2014 Dec 4. Lancet Oncol. 2015. PMID: 25481791 Clinical Trial.
-
Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial.Lancet Oncol. 2016 Nov;17(11):1579-1589. doi: 10.1016/S1470-2045(16)30376-X. Epub 2016 Sep 9. Lancet Oncol. 2016. PMID: 27617661 Clinical Trial.
-
The safety and efficacy of olaparib therapy in patients with relapsed ovarian cancer.Expert Rev Anticancer Ther. 2016 Jun;16(6):597-603. doi: 10.1080/14737140.2016.1182429. Expert Rev Anticancer Ther. 2016. PMID: 27115428 Review.
-
Spotlight on olaparib in the treatment of BRCA-mutated ovarian cancer: design, development and place in therapy.Drug Des Devel Ther. 2018 May 29;12:1501-1509. doi: 10.2147/DDDT.S124447. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29881257 Free PMC article. Review.
Cited by
-
Management of adverse events related to first-generation tyrosine receptor kinase inhibitors in adults: a narrative review.Support Care Cancer. 2022 Dec;30(12):10471-10482. doi: 10.1007/s00520-022-07401-y. Epub 2022 Nov 3. Support Care Cancer. 2022. PMID: 36326907 Review.
-
Administration of the Tablet Formulation of Olaparib in Patients with Ovarian Cancer: Practical Guidance and Expectations.Oncologist. 2018 Jun;23(6):697-703. doi: 10.1634/theoncologist.2017-0485. Epub 2018 Mar 28. Oncologist. 2018. PMID: 29593098 Free PMC article. Review.
-
PARP Inhibitors in Ovarian Cancer: A Review.Target Oncol. 2023 Jul;18(4):471-503. doi: 10.1007/s11523-023-00970-w. Epub 2023 Jun 3. Target Oncol. 2023. PMID: 37268756 Free PMC article. Review.
-
Efficacy of oral ferric citrate hydrate treatment for anemia caused by niraparib: a case report.J Med Case Rep. 2022 Nov 25;16(1):440. doi: 10.1186/s13256-022-03666-3. J Med Case Rep. 2022. PMID: 36424618 Free PMC article.
-
Tolerability of maintenance olaparib in newly diagnosed patients with advanced ovarian cancer and a BRCA mutation in the randomized phase III SOLO1 trial.Gynecol Oncol. 2021 Oct;163(1):41-49. doi: 10.1016/j.ygyno.2021.07.016. Epub 2021 Aug 2. Gynecol Oncol. 2021. PMID: 34353615 Free PMC article. Clinical Trial.
References
-
- Torre LA, Brah F, Siegel R et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. - PubMed
-
- National Cancer Institute. SEER Stat Fact Sheets: Ovary Cancer. Available at: http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 21, 2016.
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

