Switching to low-dose oral prolonged-release oxycodone/naloxone from WHO-Step I drugs in elderly patients with chronic pain at high risk of early opioid discontinuation
- PMID: 27257377
- PMCID: PMC4874636
- DOI: 10.2147/CIA.S105821
Switching to low-dose oral prolonged-release oxycodone/naloxone from WHO-Step I drugs in elderly patients with chronic pain at high risk of early opioid discontinuation
Abstract
Purpose: Chronic pain has a high prevalence in the aging population. Strong opioids also should be considered in older people for the treatment of moderate to severe pain or for pain that impairs functioning and the quality of life. This study aimed to assess the efficacy and safety of the direct switch to low-dose strong opioids (World Health Organization-Step III drugs) in elderly, opioid-naive patients.
Patients and methods: This was a single-center, retrospective, observational study in opioid-naive patients aged ≥75 years, with moderate to severe chronic pain (>6-month duration) and constipation, who initiated treatment with prolonged-release oxycodone/naloxone (OXN-PR). Patients were re-evaluated after 15, 30, and 60 days (T60, final observation). Response to treatment was defined as an improvement in pain of ≥30% after 30 days of therapy without worsening of constipation.
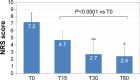
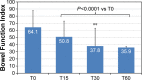
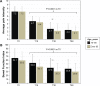
Results: One-hundred and eighty-six patients (mean ± SD age 80.7±4.7 years; 64.5% women) with severe chronic pain (mean average pain intensity 7.1±1.0 on the 11-point numerical rating scale) and constipation (mean Bowel Function Index 64.1±24.4; 89.2% of patients on laxatives) were initiated treatment with OXN-PR (mean daily dose 11.3±3.5 mg). OXN-PR reduced pain intensity rapidly and was well tolerated; 63.4% of patients responded to treatment with OXN-PR. At T60 (mean daily OXN-PR dose, 21.5±9.7 mg), the pain intensity was reduced by 66.7%. In addition, bowel function improved (mean decrease of Bowel Function Index from baseline to T60, -28.2, P<0.0001) and the use of laxatives decreased. Already after 15 days and throughout treatment, ~70% of patients perceived their status as much/extremely improved. Only 1.6% of patients discontinued treatment due to adverse events.
Conclusion: Low-dose OXN-PR in elderly patients naive to opioids proved to be an effective option for the treatment of moderate to severe chronic pain. Large-scale trials are needed to improve clinical guidance in the assessment and treatment of pain in older people.
Keywords: chronic pain; elderly; naloxone; opioid; oxycodone.
Figures

Similar articles
-
High dosage of a fixed combination oxycodone/naloxone prolonged release: efficacy and tolerability in patients with chronic cancer pain.Support Care Cancer. 2017 Oct;25(10):3051-3058. doi: 10.1007/s00520-017-3709-5. Epub 2017 May 3. Support Care Cancer. 2017. PMID: 28470370 Clinical Trial.
-
Efficacy and tolerability of low-dose oral prolonged-release oxycodone/naloxone for chronic nononcological pain in older patients.Clin Interv Aging. 2014 Dec 16;10:1-11. doi: 10.2147/CIA.S72521. eCollection 2015. Clin Interv Aging. 2014. PMID: 25565782 Free PMC article.
-
Prolonged release oxycodone and naloxone treatment counteracts opioid-induced constipation in patients with severe pain compared to previous analgesic treatment.Curr Med Res Opin. 2017 Dec;33(12):2217-2227. doi: 10.1080/03007995.2017.1367276. Epub 2017 Sep 11. Curr Med Res Opin. 2017. PMID: 28805471 Clinical Trial.
-
Opioid-Induced Constipation Relief From Fixed-Ratio Combination Prolonged-Release Oxycodone/Naloxone Compared With Oxycodone and Morphine for Chronic Nonmalignant Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.J Pain Symptom Manage. 2017 Nov;54(5):737-748.e3. doi: 10.1016/j.jpainsymman.2017.07.025. Epub 2017 Jul 21. J Pain Symptom Manage. 2017. PMID: 28736104
-
Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone.Clin Drug Investig. 2015 Jan;35(1):1-11. doi: 10.1007/s40261-014-0254-6. Clin Drug Investig. 2015. PMID: 25479959 Free PMC article. Review.
Cited by
-
Guidance on opioids prescribing for the management of persistent non-cancer pain in older adults.World J Clin Cases. 2017 Mar 16;5(3):73-81. doi: 10.12998/wjcc.v5.i3.73. World J Clin Cases. 2017. PMID: 28352631 Free PMC article. Review.
-
Opioid Prescribing in the Elderly: A Systematic Review.J Pharm Technol. 2020 Feb;36(1):28-40. doi: 10.1177/8755122519867975. Epub 2019 Aug 12. J Pharm Technol. 2020. PMID: 34752514 Free PMC article. Review.
-
Management of Opioid-Induced Constipation and Bowel Dysfunction: Expert Opinion of an Italian Multidisciplinary Panel.Adv Ther. 2021 Jul;38(7):3589-3621. doi: 10.1007/s12325-021-01766-y. Epub 2021 Jun 4. Adv Ther. 2021. PMID: 34086265 Free PMC article. Review.
-
The Effect of Oxycodone on Post-operative Pain and Inflammatory Cytokine Release in Elderly Patients Undergoing Laparoscopic Gastrectomy.Front Med (Lausanne). 2021 Sep 1;8:700025. doi: 10.3389/fmed.2021.700025. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34540861 Free PMC article.
References
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. - PubMed
-
- Abdulla A, Adams N, Bone M, et al. British Geriatric Society Guidance on the management of pain in older people. Age Ageing. 2013;42(suppl 1):i1–i57. - PubMed
-
- Chai E, Horton JR. Managing pain in the elderly population: pearls and pitfalls. Curr Pain Headache Rep. 2010;14:409–417. - PubMed
-
- Fredheim OM, Kaasa S, Fayers P, Saltnes T, Jordhøy M, Borchgrevink P. Chronic non-malignant pain patients report as poor health-related quality of life as palliative cancer patients. Acta Anaesthesiol Scand. 2008;52:143–148. - PubMed
-
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

