Similarities between decapod and insect neuropeptidomes
- PMID: 27257538
- PMCID: PMC4888303
- DOI: 10.7717/peerj.2043
Similarities between decapod and insect neuropeptidomes
Abstract
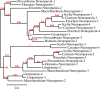
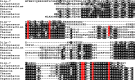
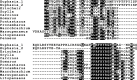
Background. Neuropeptides are important regulators of physiological processes and behavior. Although they tend to be generally well conserved, recent results using trancriptome sequencing on decapod crustaceans give the impression of significant differences between species, raising the question whether such differences are real or artefacts. Methods. The BLAST+ program was used to find short reads coding neuropeptides and neurohormons in publicly available short read archives. Such reads were then used to find similar reads in the same archives, and the DNA assembly program Trinity was employed to construct contigs encoding the neuropeptide precursors as completely as possible. Results. The seven decapod species analyzed in this fashion, the crabs Eriocheir sinensis, Carcinus maenas and Scylla paramamosain, the shrimp Litopenaeus vannamei, the lobster Homarus americanus, the fresh water prawn Macrobrachium rosenbergii and the crayfish Procambarus clarkii had remarkably similar neuropeptidomes. Although some neuropeptide precursors could not be assembled, in many cases individual reads pertaining to the missing precursors show unambiguously that these neuropeptides are present in these species. In other cases, the tissues that express those neuropeptides were not used in the construction of the cDNA libraries. One novel neuropeptide was identified: elongated PDH (pigment dispersing hormone), a variation on PDH that has a two-amino-acid insertion in its core sequence. Hyrg is another peptide that is ubiquitously present in decapods and is likely a novel neuropeptide precursor. Discussion. Many insect species have lost one or more neuropeptide genes, but apart from elongated PDH and hyrg all other decapod neuropeptides are present in at least some insect species, and allatotropin is the only insect neuropeptide missing from decapods. This strong similarity between insect and decapod neuropeptidomes makes it possible to predict the receptors for decapod neuropeptides that have been deorphanized in insects. This includes the androgenic insulin-like peptide that seems to be homologous to drosophila insulin-like peptide 8.
Keywords: Agatoxin-like peptide; Androgenic insulin-like peptide; Calcitonin; Crustacean female sex hormone; Evolution; Neuroparsin; Neuropeptide; PDH; Receptor.
Conflict of interest statement
The author declares there are no competing interests.
Figures









References
-
- Aizen J, Chandler JC, Fitzgibbon QP, Sagi A, Battaglene SC, Elizur A, Ventura T. Production of recombinant insulin-like androgenic gland hormones from three decapod species: in vitro testicular phosphorylation and activation of a newly identified tyrosine kinase receptor from the Eastern spiny lobster, Sagmariasus verreauxi. General and Comparative Endocrinology. 2016;229:8–18. doi: 10.1016/j.ygcen.2016.02.013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

