Raptor/mTORC1 loss in adipocytes causes progressive lipodystrophy and fatty liver disease
- PMID: 27257602
- PMCID: PMC4877665
- DOI: 10.1016/j.molmet.2016.04.001
Raptor/mTORC1 loss in adipocytes causes progressive lipodystrophy and fatty liver disease
Abstract
Objective: Normal adipose tissue growth and function is critical to maintaining metabolic homeostasis and its excess (e.g. obesity) or absence (e.g. lipodystrophy) is associated with severe metabolic disease. The goal of this study was to understand the mechanisms maintaining healthy adipose tissue growth and function.
Methods: Adipose tissue senses and responds to systemic changes in growth factor and nutrient availability; in cells mTORC1 regulates metabolism in response to growth factors and nutrients. Thus, mTORC1 is poised to be a critical intracellular regulator of adipocyte metabolism. Here, we investigate the role of mTORC1 in mature adipocytes by generating and characterizing mice in which the Adiponectin-Cre driver is used to delete floxed alleles of Raptor, which encodes an essential regulatory subunit of mTORC1.
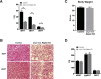
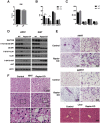
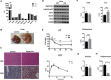
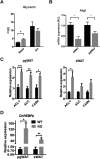
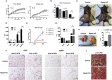
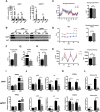
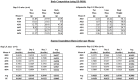
Results: Raptor (Adipoq-cre) mice have normal white adipose tissue (WAT) mass for the first few weeks of life, but soon thereafter develop lipodystrophy associated with hepatomegaly, hepatic steatosis, and insulin intolerance. Raptor (Adipoq-cre) mice are also resistant to becoming obese when consuming a high fat diet (HFD). Resistance to obesity does not appear to be due to increased energy expenditure, but rather from failed adipose tissue expansion resulting in severe hepatomegaly associated with hyperphagia and defective dietary lipid absorption. Deleting Raptor in WAT also decreases C/EBPα expression and the expression of its downstream target adiponectin, providing one possible mechanism of mTORC1 function in WAT.
Conclusions: mTORC1 activity in mature adipocytes is essential for maintaining normal adipose tissue growth and its selective loss in mature adipocytes leads to a progressive lipodystrophy disorder and systemic metabolic disease that shares many of the hallmarks of human congenital generalized lipodystrophy.
Keywords: Lipodystrophy; Obesity; Rapamycin; Raptor; White Adipose Tissue (WAT); mTORC1.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

