Multiscale Aspects of Generation of High-Gamma Activity during Seizures in Human Neocortex
- PMID: 27257623
- PMCID: PMC4876490
- DOI: 10.1523/ENEURO.0141-15.2016
Multiscale Aspects of Generation of High-Gamma Activity during Seizures in Human Neocortex
Abstract
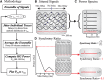
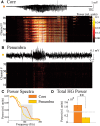
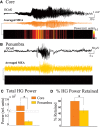
High-gamma (HG; 80-150 Hz) activity in macroscopic clinical records is considered a marker for critical brain regions involved in seizure initiation; it is correlated with pathological multiunit firing during neocortical seizures in the seizure core, an area identified by correlated multiunit spiking and low frequency seizure activity. However, the effects of the spatiotemporal dynamics of seizure on HG power generation are not well understood. Here, we studied HG generation and propagation, using a three-step, multiscale signal analysis and modeling approach. First, we analyzed concurrent neuronal and microscopic network HG activity in neocortical slices from seven intractable epilepsy patients. We found HG activity in these networks, especially when neurons displayed paroxysmal depolarization shifts and network activity was highly synchronized. Second, we examined HG activity acquired with microelectrode arrays recorded during human seizures (n = 8). We confirmed the presence of synchronized HG power across microelectrode records and the macroscale, both specifically associated with the core region of the seizure. Third, we used volume conduction-based modeling to relate HG activity and network synchrony at different network scales. We showed that local HG oscillations require high levels of synchrony to cross scales, and that this requirement is met at the microscopic scale, but not within macroscopic networks. Instead, we present evidence that HG power at the macroscale may result from harmonics of ongoing seizure activity. Ictal HG power marks the seizure core, but the generating mechanism can differ across spatial scales.
Keywords: HFOs; epilepsy; human; modeling; neocortex; seizure.
Figures





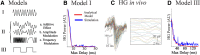
References
-
- Berg A, Berkovic S, Brodie M, Buchhalter J, Cross J, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology 2005-2009. Epilepsia 51:676–685. 10.1111/j.1528-1167.2010.02522.x - DOI - PubMed
-
- Bragin A, Engel J Jr, Wilson C, Fried I, Buzsáki G (1999a) High-frequency oscillations in human brain. Hippocampus 9:137–142. - PubMed
-
- Bragin A, Engel J Jr, Wilson C, Fried I, Mathern G (1999b) Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid–treated rats with chronic seizures. Epilepsia 40:127–137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
