c-Jun N-Terminal Phosphorylation: Biomarker for Cellular Stress Rather than Cell Death in the Injured Cochlea
- PMID: 27257624
- PMCID: PMC4877566
- DOI: 10.1523/ENEURO.0047-16.2016
c-Jun N-Terminal Phosphorylation: Biomarker for Cellular Stress Rather than Cell Death in the Injured Cochlea
Abstract
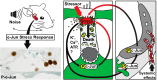
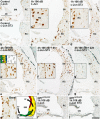
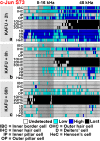
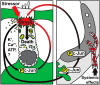
Prevention of auditory hair cell death offers therapeutic potential to rescue hearing. Pharmacological blockade of JNK/c-Jun signaling attenuates injury-induced hair cell loss, but with unsolved mechanisms. We have characterized the c-Jun stress response in the mouse cochlea challenged with acoustic overstimulation and ototoxins, by studying the dynamics of c-Jun N-terminal phosphorylation. It occurred acutely in glial-like supporting cells, inner hair cells, and the cells of the cochlear ion trafficking route, and was rapidly downregulated after exposures. Notably, death-prone outer hair cells lacked c-Jun phosphorylation. As phosphorylation was triggered also by nontraumatic noise levels and none of the cells showing this activation were lost, c-Jun phosphorylation is a biomarker for cochlear stress rather than an indicator of a death-prone fate of hair cells. Preconditioning with a mild noise exposure before a stronger traumatizing noise exposure attenuated the cochlear c-Jun stress response, suggesting that the known protective effect of sound preconditioning on hearing is linked to suppression of c-Jun activation. Finally, mice with mutations in the c-Jun N-terminal phosphoacceptor sites showed partial, but significant, hair cell protection. These data identify the c-Jun stress response as a paracrine mechanism that mediates outer hair cell death.
Keywords: c-Jun phosphorylation; cell death; hair cell; inner ear; noise; supporting cell.
Conflict of interest statement
The authors declare no conflicting financial interests.
Figures








References
-
- Behrens A, Sibilia M, Wagner EF (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet 21:326-329. http://www.nature.com/ng/journal/v21/n3/full/ng0399_326.html - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
