Quantification and Comparison of Anti-Fibrotic Therapies by Polarized SRM and SHG-Based Morphometry in Rat UUO Model
- PMID: 27257917
- PMCID: PMC4892485
- DOI: 10.1371/journal.pone.0156734
Quantification and Comparison of Anti-Fibrotic Therapies by Polarized SRM and SHG-Based Morphometry in Rat UUO Model
Abstract
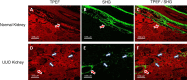
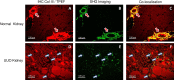
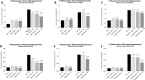
Renal interstitial fibrosis (IF) is an important pathologic manifestation of disease progression in a variety of chronic kidney diseases (CKD). However, the quantitative and reproducible analysis of IF remains a challenge, especially in experimental animal models of progressive IF. In this study, we compare traditional polarized Sirius Red morphometry (SRM) to novel Second Harmonic Generation (SHG)-based morphometry of unstained tissues for quantitative analysis of IF in the rat 5 day unilateral ureteral obstruction (UUO) model. To validate the specificity of SHG for detecting fibrillar collagen components in IF, co-localization studies for collagens type I, III, and IV were performed using IHC. In addition, we examined the correlation, dynamic range, sensitivity, and ability of polarized SRM and SHG-based morphometry to detect an anti-fibrotic effect of three different treatment regimens. Comparisons were made across three separate studies in which animals were treated with three mechanistically distinct pharmacologic agents: enalapril (ENA, 15, 30, 60 mg/kg), mycophenolate mofetil (MMF, 2, 20 mg/kg) or the connective tissue growth factor (CTGF) neutralizing antibody, EX75606 (1, 3, 10 mg/kg). Our results demonstrate a strong co-localization of the SHG signal with fibrillar collagens I and III but not non-fibrillar collagen IV. Quantitative IF, calculated as percent cortical area of fibrosis, demonstrated similar response profile for both polarized SRM and SHG-based morphometry. The two methodologies exhibited a strong correlation across all three pharmacology studies (r2 = 0.89-0.96). However, compared with polarized SRM, SHG-based morphometry delivered a greater dynamic range and absolute magnitude of reduction of IF after treatment. In summary, we demonstrate that SHG-based morphometry in unstained kidney tissues is comparable to polarized SRM for quantitation of fibrillar collagens, but with an enhanced sensitivity to detect treatment-induced reductions in IF. Thus, performing SHG-based morphometry on unstained kidney tissue is a reliable alternative to traditional polarized SRM for quantitative analysis of IF.
Conflict of interest statement
Figures


References
-
- Liu Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 2006; 69: 213–217 - PubMed
-
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979; 11: 447–455 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

