Development of a large peptoid-DOTA combinatorial library
- PMID: 27257968
- PMCID: PMC5035194
- DOI: 10.1002/bip.22883
Development of a large peptoid-DOTA combinatorial library
Abstract
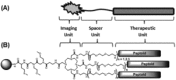
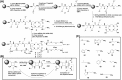
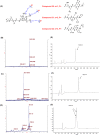
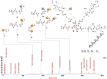
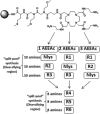
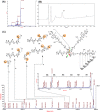
Conventional one-bead one-compound (OBOC) library synthesis is typically used to identify molecules with therapeutic value. The design and synthesis of OBOC libraries that contain molecules with imaging or even potentially therapeutic and diagnostic capacities (e.g. theranostic agents) has been overlooked. The development of a therapeutically active molecule with a built-in imaging component for a certain target is a daunting task, and structure-based rational design might not be the best approach. We hypothesize to develop a combinatorial library with potentially therapeutic and imaging components fused together in each molecule. Such molecules in the library can be used to screen, identify, and validate as direct theranostic candidates against targets of interest. As the first step in achieving that aim, we developed an on-bead library of 153,600 Peptoid-DOTA compounds in which the peptoids are the target-recognizing and potentially therapeutic components and the DOTA is the imaging component. We attached the DOTA scaffold to TentaGel beads using one of the four arms of DOTA, and we built a diversified 6-mer peptoid library on the remaining three arms. We evaluated both the synthesis and the mass spectrometric sequencing capacities of the test compounds and of the final library. The compounds displayed unique ionization patterns including direct breakages of the DOTA scaffold into two units, allowing clear decoding of the sequences. Our approach provides a facile synthesis method for the complete on-bead development of large peptidomimetic-DOTA libraries for screening against biological targets for the identification of potential theranostic agents in the future. © 2016 The Authors. Biopolymers Published by Wiley Periodicals, Inc. Biopolymers (Pept Sci) 106: 673-684, 2016.
Keywords: DOTA; combinatorial; imaging; peptoids; theranostics.
© 2016 The Authors. Biopolymers Published by Wiley Periodicals, Inc.
Figures

References
-
- Lam, K. S. ; Salmon, S. E. ; Hersh, E. M. ; Hruby, V. J. ; Kazmierski, W. M. ; Knapp, R. J. Nature 1991, 354, 82–84. - PubMed
-
- Furka, A. ; Sebestyen, F. ; Asgedom, M. ; Dibo, G. Int J Pept Protein Res 1991, 37, 487–493. - PubMed
-
- Lebl, M. ; Krchnak, V. ; Sepetov, N. F. ; Seligmann, B. ; Strop, P. ; Felder, S. ; Lam, K. S. Biopolymers 1995, 37, 177–198. - PubMed
-
- Lam, K. S. ; Lebl, M. ; Krchnak, V. Chem Rev 1997, 97, 411–448. - PubMed
-
- Bononi, F. C. ; Luyt, L. G. Methods Mol. Biol 2015, 1248, 223–237. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

