Differentiation of Murine Bone Marrow-Derived Smooth Muscle Progenitor Cells Is Regulated by PDGF-BB and Collagen
- PMID: 27258003
- PMCID: PMC4892566
- DOI: 10.1371/journal.pone.0156935
Differentiation of Murine Bone Marrow-Derived Smooth Muscle Progenitor Cells Is Regulated by PDGF-BB and Collagen
Abstract
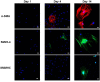
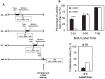
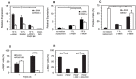
Smooth muscle cells (SMCs) are key regulators of vascular disease and circulating smooth muscle progenitor cells may play important roles in vascular repair or remodelling. We developed enhanced protocols to derive smooth muscle progenitors from murine bone marrow and tested whether factors that are increased in atherosclerotic plaques, namely platelet-derived growth factor-BB (PDGF-BB) and monomeric collagen, can influence the smooth muscle specific differentiation, proliferation, and survival of mouse bone marrow-derived progenitor cells. During a 21 day period of culture, bone marrow cells underwent a marked increase in expression of the SMC markers α-SMA (1.93 ± 0.15 vs. 0.0008 ± 0.0003 (ng/ng GAPDH) at 0 d), SM22-α (1.50 ± 0.27 vs. 0.005 ± 0.001 (ng/ng GAPDH) at 0 d) and SM-MHC (0.017 ± 0.004 vs. 0.001 ± 0.001 (ng/ng GAPDH) at 0 d). Bromodeoxyuridine (BrdU) incorporation experiments showed that in early culture, the smooth muscle progenitor subpopulation could be identified by high proliferative rates prior to the expression of smooth muscle specific markers. Culture of fresh bone marrow or smooth muscle progenitor cells with PDGF-BB suppressed the expression of α-SMA and SM22-α, in a rapidly reversible manner requiring PDGF receptor kinase activity. Progenitors cultured on polymerized collagen gels demonstrated expression of SMC markers, rates of proliferation and apoptosis similar to that of cells on tissue culture plastic; in contrast, cells grown on monomeric collagen gels displayed lower SMC marker expression, lower growth rates (319 ± 36 vs. 635 ± 97 cells/mm2), and increased apoptosis (5.3 ± 1.6% vs. 1.0 ± 0.5% (Annexin 5 staining)). Our data shows that the differentiation and survival of smooth muscle progenitors are critically affected by PDGF-BB and as well as the substrate collagen structure.
Conflict of interest statement
Figures




Similar articles
-
Impact of bladder-derived acellular matrix, growth factors, and extracellular matrix constituents on the survival and multipotency of marrow-derived mesenchymal stem cells.J Biomed Mater Res A. 2012 Jan;100(1):72-83. doi: 10.1002/jbm.a.33230. Epub 2011 Oct 4. J Biomed Mater Res A. 2012. PMID: 21972045
-
Platelet-derived growth factor-BB (PDGF-BB) induces differentiation of bone marrow endothelial progenitor cell-derived cell line TR-BME2 into mural cells, and changes the phenotype.J Cell Physiol. 2005 Sep;204(3):948-55. doi: 10.1002/jcp.20362. J Cell Physiol. 2005. PMID: 15828021
-
[PDGF-BB initiates vascular smooth muscle-like phenotype differentiation of human bone marrow mesenchymal stem cells in vitro].Zhonghua Zheng Xing Wai Ke Za Zhi. 2007 Jul;23(4):335-9. Zhonghua Zheng Xing Wai Ke Za Zhi. 2007. PMID: 17926862 Chinese.
-
Circulating smooth muscle progenitor cells in arterial remodeling.J Mol Cell Cardiol. 2011 Feb;50(2):273-9. doi: 10.1016/j.yjmcc.2010.10.030. Epub 2010 Nov 1. J Mol Cell Cardiol. 2011. PMID: 21047514 Review.
-
Smooth muscle progenitor cells: friend or foe in vascular disease?Curr Stem Cell Res Ther. 2009 May;4(2):131-40. doi: 10.2174/157488809788167454. Curr Stem Cell Res Ther. 2009. PMID: 19442197 Free PMC article. Review.
Cited by
-
HDACs regulate the differentiation of endothelial cells from human iPSCs.Cell Biochem Funct. 2022 Aug;40(6):589-599. doi: 10.1002/cbf.3729. Epub 2022 Jul 5. Cell Biochem Funct. 2022. PMID: 35789099 Free PMC article.
-
Vascular precursor cells in tissue injury repair.Transl Res. 2017 Jun;184:77-100. doi: 10.1016/j.trsl.2017.02.002. Epub 2017 Feb 21. Transl Res. 2017. PMID: 28284670 Free PMC article. Review.
-
Modified method for effective primary vascular smooth muscle progenitor cell culture from peripheral blood.Cytotechnology. 2020 Oct;72(5):763-772. doi: 10.1007/s10616-020-00419-2. Epub 2020 Sep 9. Cytotechnology. 2020. PMID: 32909140 Free PMC article.
References
-
- Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26(12):2696–702. Epub 2006/09/30. 10.1161/01.ATV.0000247243.48542.9d . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

