Increased Expression of Readthrough Acetylcholinesterase Variants in the Brains of Alzheimer's Disease Patients
- PMID: 27258420
- PMCID: PMC5013723
- DOI: 10.3233/JAD-160220
Increased Expression of Readthrough Acetylcholinesterase Variants in the Brains of Alzheimer's Disease Patients
Abstract
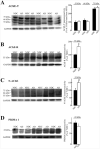
Alzheimer's disease (AD) is characterized by a decrease in the enzymatic activity of the enzyme acetylcholinesterase (AChE). AChE is expressed as multiple splice variants, which may serve both cholinergic degradative functions and non-cholinergic functions unrelated with their capacity to hydrolyze acetylcholine. We have recently demonstrated that a prominent pool of enzymatically inactive AChE protein exists in the AD brain. In this study, we analyzed protein and transcript levels of individual AChE variants in human frontal cortex from AD patients by western blot analysis using specific anti-AChE antibodies and by quantitative real-time PCR (qRT-PCR). We found similar protein and mRNA levels of the major cholinergic "tailed"-variant (AChE-T) and the anchoring subunit, proline-rich membrane anchor (PRiMA-1) in frontal cortex obtained from AD patients and non-demented controls. Interestingly, we found an increase in the protein and transcript levels of the non-cholinergic "readthrough" AChE (AChE-R) variants in AD patients compared to controls. Similar increases were detected by western blot using an antibody raised against the specific N-terminal domain, exclusive of alternative N-extended variants of AChE (N-AChE). In accordance with a subset of AChE-R monomers that display amphiphilic properties that are upregulated in the AD brain, we demonstrate that the increase of N-AChE species is due, at least in part, to N-AChE-R variants. In conclusion, we demonstrate selective alterations in specific AChE variants in AD cortex, with no correlation in enzymatic activity. Therefore, differential expression of AChE variants in AD may reflect changes in the pathophysiological role of AChE, independent of cholinergic impairment or its role in degrading acetylcholine.
Keywords: AChE splice variants; Acetylcholinesterase; Alzheimer’s disease; human brain; readthrough AChE variants.
Figures


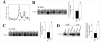
References
-
- Alzheimer's Association*. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. - PubMed
-
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delong MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. - PubMed
-
- Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer's disease. Am J Geriatr Psychiatry. 1998;6:S64–78. - PubMed
-
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

