Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth
- PMID: 27258787
- PMCID: PMC5041190
- DOI: 10.1038/cdd.2016.48
Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth
Abstract
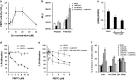
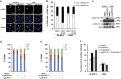
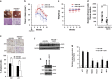
Mutations in the p53 tumor-suppressor gene are prevalent in human cancers. The majority of p53 mutations are missense, which can be classified into contact mutations (that directly disrupts the DNA-binding activity of p53) and structural mutations (that disrupts the conformation of p53). Both of the mutations can disable the normal wild-type (WT) p53 activities. Nevertheless, it has been amply documented that small molecules can rescue activity from mutant p53 by restoring WT tumor-suppressive functions. These compounds hold promise for cancer therapy and have now entered clinical trials. In this study, we show that cruciferous-vegetable-derived phenethyl isothiocyanate (PEITC) can reactivate p53 mutant under in vitro and in vivo conditions, revealing a new mechanism of action for a dietary-related compound. PEITC exhibits growth-inhibitory activity in cells expressing p53 mutants with preferential activity toward p53(R175), one of the most frequent 'hotspot' mutations within the p53 sequence. Mechanistic studies revealed that PEITC induces apoptosis in a p53(R175) mutant-dependent manner by restoring p53 WT conformation and transactivation functions. Accordingly, in PEITC-treated cells the reactivated p53(R175) mutant induces apoptosis by activating canonical WT p53 targets, inducing a delay in S and G2/M phase, and by phosphorylating ATM/CHK2. Interestingly, the growth-inhibitory effects of PEITC depend on the redox state of the cell. Further, PEITC treatments render the p53(R175) mutant sensitive to degradation by the proteasome and autophagy in a concentration-dependent manner. PEITC-induced reactivation of p53(R175) and its subsequent sensitivity to the degradation pathways likely contribute to its anticancer activities. We further show that dietary supplementation of PEITC is able to reactivate WT activity in vivo as well, inhibiting tumor growth in xenograft mouse model. These findings provide the first example of mutant p53 reactivation by a dietary compound and have important implications for cancer prevention and therapy.
Figures





References
-
- Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139: 1327–1341. - PubMed
-
- Dittmer D, Pati S, Zambetti G, Chu S, Teresky AK, Moore M et al. Gain of function mutations in p53. Nat Genet 1993; 4: 42–46. - PubMed
-
- Hisada M, Garber JE, Fung CY, Fraumeni JF Jr, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90: 606–611. - PubMed
-
- WHOIARC Handbook on Cancer Prevention. Cruciferous Vegetables, Isothiocyanates and Indoles, vol. 9. IARC Press: Lyon, France, 2004.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

