Ataxia-Pancytopenia Syndrome Is Caused by Missense Mutations in SAMD9L
- PMID: 27259050
- PMCID: PMC4908176
- DOI: 10.1016/j.ajhg.2016.04.009
Ataxia-Pancytopenia Syndrome Is Caused by Missense Mutations in SAMD9L
Abstract
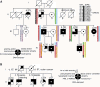
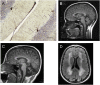
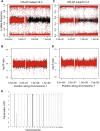
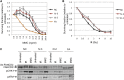
Ataxia-pancytopenia (AP) syndrome is characterized by cerebellar ataxia, variable hematologic cytopenias, and predisposition to marrow failure and myeloid leukemia, sometimes associated with monosomy 7. Here, in the four-generation family UW-AP, linkage analysis revealed four regions that provided the maximal LOD scores possible, one of which was in a commonly microdeleted chromosome 7q region. Exome sequencing identified a missense mutation (c.2640C>A, p.His880Gln) in the sterile alpha motif domain containing 9-like gene (SAMD9L) that completely cosegregated with disease. By targeted sequencing of SAMD9L, we subsequently identified a different missense mutation (c.3587G>C, p.Cys1196Ser) in affected members of the first described family with AP syndrome, Li-AP. Neither variant is reported in the public databases, both affect highly conserved amino acid residues, and both are predicted to be damaging. With time in culture, lymphoblastic cell lines (LCLs) from two affected individuals in family UW-AP exhibited copy-neutral loss of heterozygosity for large portions of the long arm of chromosome 7, resulting in retention of only the wild-type SAMD9L allele. Newly established LCLs from both individuals demonstrated the same phenomenon. In addition, targeted capture and sequencing of SAMD9L in uncultured blood DNA from both individuals showed bias toward the wild-type allele. These observations indicate in vivo hematopoietic mosaicism. The hematopoietic cytopenias that characterize AP syndrome and the selective advantage for clones that have lost the mutant allele support the postulated role of SAMD9L in the regulation of cell proliferation. Furthermore, we show that AP syndrome is distinct from the dyskeratoses congenita telomeropathies, with which it shares some clinical characteristics.
Copyright © 2016 American Society of Human Genetics. All rights reserved.
Figures

References
-
- Li F.P., Potter N.U., Buchanan G.R., Vawter G., Whang-Peng J., Rosen R.B. A family with acute leukemia, hypoplastic anemia and cerebellar ataxia: association with bone marrow C-monosomy. Am. J. Med. 1978;65:933–940. - PubMed
-
- Li F.P., Hecht F., Kaiser-McCaw B., Baranko P.V., Potter N.U. Ataxia-pancytopenia: syndrome of cerebellar ataxia, hypoplastic anemia, monosomy 7, and acute myelogenous leukemia. Cancer Genet. Cytogenet. 1981;4:189–196. - PubMed
-
- Daghistani D., Curless R., Toledano S.R., Ayyar D.R. Ataxia-pancytopenia and monosomy 7 syndrome. J. Pediatr. 1989;115:108–110. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U54 HG006493/HG/NHGRI NIH HHS/United States
- UC2 HL102926/HL/NHLBI NIH HHS/United States
- UC2 HL103010/HL/NHLBI NIH HHS/United States
- R24 DK099808/DK/NIDDK NIH HHS/United States
- RC2 HG005608/HG/NHGRI NIH HHS/United States
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- R01 NS069719/NS/NINDS NIH HHS/United States
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- UC2 HL102924/HL/NHLBI NIH HHS/United States
- UM1 HG006493/HG/NHGRI NIH HHS/United States
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- I01 CX001006/CX/CSRD VA/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
- UC2 HL102925/HL/NHLBI NIH HHS/United States
- RA/ARRA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

