Therapeutic Drug Monitoring, Electronic Health Records, and Pharmacokinetic Modeling to Evaluate Sirolimus Drug Exposure-Response Relationships in Renal Transplant Patients
- PMID: 27259059
- PMCID: PMC5025355
- DOI: 10.1097/FTD.0000000000000313
Therapeutic Drug Monitoring, Electronic Health Records, and Pharmacokinetic Modeling to Evaluate Sirolimus Drug Exposure-Response Relationships in Renal Transplant Patients
Abstract
Background: Sirolimus, an immunosuppressive agent used in renal transplantation, can prevent allograft rejection. Identification of the therapeutic index (the ratio of minimum toxic concentration to minimum therapeutic concentration) for immunosuppresants is necessary to optimize the care of patients and set standards for bioequivalence evaluation of sirolimus products. However, the therapeutic index for sirolimus has been inconsistently defined, potentially because of inconsistencies in sirolimus exposure-response relationships.
Methods: The authors used retrospective therapeutic drug monitoring data from the electronic health records of patients treated in a tertiary health care system from 2008 to 2014 to (1) develop a population pharmacokinetic (PK) model, (2) use the model to simulate sirolimus concentrations, and (3) characterize the exposure-response relationship. Using Wilcoxon rank-sum and Fisher exact tests, the authors determined relationships between sirolimus exposure and adverse events (AEs) (anemia, leukopenia, thrombocytopenia, hyperlipidemia, and decline in renal function) and the composite efficacy end point of graft loss or rejection.
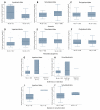
Results: The developed 2-compartment population PK model showed appropriate goodness of fit. In a late-phase (>12 months), postrenal transplant population of 27 inpatients, the authors identified statistically significant relationships between 83 simulated peak and trough sirolimus concentrations and outcomes: graft loss or rejection (P = 0.018) and decline in renal function (P = 0.006), respectively.
Conclusions: Use of therapeutic drug monitoring results and PK modeling permitted correlation of sirolimus concentrations with graft loss or rejection and decline in renal function. However, the method was limited in its assessment of other AEs. To better evaluate sirolimus exposure-response relationships, the method should be applied to a larger sample of newly transplanted patients with a higher propensity toward AEs or efficacy failure.
Figures
Similar articles
-
Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity.Clin Transplant. 2000 Apr;14(2):97-109. doi: 10.1034/j.1399-0012.2000.140201.x. Clin Transplant. 2000. PMID: 10770413
-
Requirements for therapeutic drug monitoring of sirolimus, an immunosuppressive agent used in renal transplantation.Clin Ther. 2000;22 Suppl B:B86-92. doi: 10.1016/s0149-2918(00)89025-6. Clin Ther. 2000. PMID: 10823376 Review.
-
Population pharmacokinetics and pharmacogenetics of everolimus in renal transplant patients.Clin Pharmacokinet. 2012 Jul 1;51(7):467-80. doi: 10.2165/11599710-000000000-00000. Clin Pharmacokinet. 2012. PMID: 22624503
-
Sirolimus population pharmacokinetic/pharmacogenetic analysis and bayesian modelling in kidney transplant recipients.Clin Pharmacokinet. 2006;45(11):1135-48. doi: 10.2165/00003088-200645110-00007. Clin Pharmacokinet. 2006. PMID: 17048977
-
Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus.Clin Ther. 2000;22 Suppl B:B101-121. doi: 10.1016/s0149-2918(00)89027-x. Clin Ther. 2000. PMID: 10823378 Review.
Cited by
-
Coadministration of fluconazole to boost subtherapeutic sirolimus concentrations: A case report.Pharmacol Res Perspect. 2024 Jun;12(3):e1198. doi: 10.1002/prp2.1198. Pharmacol Res Perspect. 2024. PMID: 38635290 Free PMC article.
-
Analysis of Sirolimus Blood Concentration and Influencing Factors in Pediatric Patients: Implications for Individualized Drug Therapy.Drugs R D. 2025 Mar;25(1):79-88. doi: 10.1007/s40268-025-00506-9. Epub 2025 Apr 11. Drugs R D. 2025. PMID: 40214962 Free PMC article.
-
Effects of CYP3A5 Genetic Polymorphisms on the Weight-adjusted through Concentration of Sirolimus in Renal Transplant Recipients: A Systematic Review and Meta-analysis.Curr Pharm Des. 2024;30(39):3108-3115. doi: 10.2174/0113816128324199240730093415. Curr Pharm Des. 2024. PMID: 39171589
-
External Evaluation of a Gentamicin Infant Population Pharmacokinetic Model Using Data from a National Electronic Health Record Database.Antimicrob Agents Chemother. 2018 Aug 27;62(9):e00669-18. doi: 10.1128/AAC.00669-18. Print 2018 Sep. Antimicrob Agents Chemother. 2018. PMID: 29914947 Free PMC article.
References
-
- Report of the Committee for the Assessment of Biometric Aspects of Controlled Trials of Hypoglycemic Agents. JAMA. 1975;231:583–608. - PubMed
-
- Kuypers DR. Benefit-risk assessment of sirolimus in renal transplantation. Drug Saf. 2005;28(2):153–181. - PubMed
-
- [Accessed August 1, 2006];EuroQol Web. 2006 http://gs1.q4matics.com/EuroqolPublishWeb/
-
- Kahan BD, Napoli KL, Kelly PA, et al. Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity. Clin Transplant. 2000 Apr;14(2):97–109. - PubMed
-
- Kelly PA, Napoli K, Kahan BD. Conversion from liquid to solid rapamycin formulations in stable renal allograft transplant recipients. Biopharmaceutics & drug disposition. 1999 Jul;20(5):249–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 HD083465/HD/NICHD NIH HHS/United States
- U01 FD004858/FD/FDA HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- K23 NS085049/NS/NINDS NIH HHS/United States
- KL2 TR001115/TR/NCATS NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- K12 HD043494/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- HHSN272201500006C/AI/NIAID NIH HHS/United States
- R01 HD076676/HD/NICHD NIH HHS/United States
- T32 HD043029/HD/NICHD NIH HHS/United States
- HHSN272201300017C/AI/NIAID NIH HHS/United States
- HHSN272201300017I/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

