The impact of 177Lu-octreotide therapy on 99mTc-MAG3 clearance is not predictive for late nephropathy
- PMID: 27259246
- PMCID: PMC5173054
- DOI: 10.18632/oncotarget.9775
The impact of 177Lu-octreotide therapy on 99mTc-MAG3 clearance is not predictive for late nephropathy
Abstract
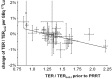
Peptide Receptor Radionuclide Therapy (PRRT) for the treatment of neuroendocrine tumors may lead to kidney deterioration. This study aimed to evaluate the suitability of 99mTc-mercaptoacetyltriglycine (99mTc--MAG3) clearance for the early detection of PRRT-induced changes on tubular extraction (TE). TE rate (TER) was measured prior to 128 PRRT cycles (7.6±0.4 GBq 177Lu-octreotate/octreotide each) in 32 patients. TER reduction during PRRT was corrected for age-related decrease and analyzed for the potential to predict loss of glomerular filtration (GF). The GF rate (GFR) as measure for renal function was derived from serum creatinine. The mean TER was 234 ± 53 ml/min/1.73 m² before PRRT (baseline) and 221 ± 45 ml/min/1.73 m² after a median follow-up of 370 days. The age-corrected decrease (mean: -3%, range: -27% to +19%) did not reach significance (p=0.09) but significantly correlated with the baseline TER (Spearman p=-0.62, p<0.001). Patients with low baseline TER showed an improved TER after PRRT, high decreases were only observed in individuals with high baseline TER. Pre-therapeutic TER data were inferior to plasma creatinine-derived GFR estimates in predicting late nephropathy. TER assessed by 99mTc-MAG3-clearance prior to and during PRRT is not suitable as early predictor of renal injury and an increased risk for late nephropathy.
Keywords: 177Lu; MAG3; PRRT; neuroendocrine tumor; renal scintigraphy.
Conflict of interest statement
CONFLICTS OF INTERESTS RAW has received the “YING” research grant from Novartis Pharma, Nürnberg, Germany. AW has received the “YING” research grant from Novartis Pharma, Nürnberg, Germany. All other authors declare no conflict of interests.
Figures


Similar articles
-
Dynamic and static small-animal SPECT in rats for monitoring renal function after 177Lu-labeled Tyr3-octreotate radionuclide therapy.J Nucl Med. 2010 Dec;51(12):1962-8. doi: 10.2967/jnumed.110.080143. Epub 2010 Nov 15. J Nucl Med. 2010. PMID: 21078795
-
177Lu-DOTATATE PRRT in Patients with Metastatic Neuroendocrine Tumor and a Single Functioning Kidney: Tolerability and Effect on Renal Function.J Nucl Med Technol. 2016 Jun;44(2):65-9. doi: 10.2967/jnmt.115.168146. Epub 2016 Feb 4. J Nucl Med Technol. 2016. PMID: 26848166
-
Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate.Eur J Nucl Med Mol Imaging. 2014 Mar;41(3):505-10. doi: 10.1007/s00259-013-2601-x. Epub 2013 Nov 6. Eur J Nucl Med Mol Imaging. 2014. PMID: 24196919
-
Renal Function Assessment During Peptide Receptor Radionuclide Therapy.Semin Nucl Med. 2016 Sep;46(5):462-78. doi: 10.1053/j.semnuclmed.2016.04.006. Semin Nucl Med. 2016. PMID: 27553471 Review.
-
Myelotoxicity of Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: A Decade of Experience.Cancer Biother Radiopharm. 2016 Aug;31(6):189-98. doi: 10.1089/cbr.2016.2035. Epub 2016 Jul 15. Cancer Biother Radiopharm. 2016. PMID: 27419665 Review.
Cited by
-
Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity.Oncotarget. 2017 Jan 24;8(4):7039-7049. doi: 10.18632/oncotarget.12402. Oncotarget. 2017. PMID: 27705948 Free PMC article. Clinical Trial.
-
Current and future perspectives on functional molecular imaging in nephro-urology: theranostics on the horizon.Theranostics. 2021 Apr 7;11(12):6105-6119. doi: 10.7150/thno.58682. eCollection 2021. Theranostics. 2021. PMID: 33897902 Free PMC article. Review.
-
Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity.Eur J Nucl Med Mol Imaging. 2021 Dec;49(1):54-63. doi: 10.1007/s00259-021-05474-1. Epub 2021 Aug 26. Eur J Nucl Med Mol Imaging. 2021. PMID: 34448031 Free PMC article.
-
The increasing potential of nuclear medicine imaging for the evaluation and reduction of normal tissue toxicity from radiation treatments.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):3762-3775. doi: 10.1007/s00259-021-05284-5. Epub 2021 Mar 9. Eur J Nucl Med Mol Imaging. 2021. PMID: 33687522 Free PMC article. Review.
-
The Dependence of Renal 68Ga[Ga]-DOTATOC Uptake on Kidney Function and Its Relevance for Peptide Receptor Radionuclide Therapy with 177Lu[Lu]-DOTATOC.Diagnostics (Basel). 2021 Jul 6;11(7):1216. doi: 10.3390/diagnostics11071216. Diagnostics (Basel). 2021. PMID: 34359299 Free PMC article.
References
-
- Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Mittra E, Hobday T, Hendifar A, Oberg K, Sierra ML, Ruszniewski P, Kwekkeboom D. 177-Lu-Dotatate significantly improves progression-free survival in patients with midgut neuroendocrine tumours: Results of the phase III NETTER-1 trial. European Journal of Cancer. 2015;51:S710–S710.
-
- Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. - PubMed
-
- Lapa C, Werner RA, Schmid JS, Papp L, Zsoter N, Biko J, Reiners C, Herrmann K, Buck AK, Bundschuh RA. Prognostic value of positron emission tomography-assessed tumor heterogeneity in patients with thyroid cancer undergoing treatment with radiopeptide therapy. Nucl Med Biol. 2015;42:349–354. - PubMed
-
- Strigari L, Konijnenberg M, Chiesa C, Bardies M, Du Y, Gleisner KS, Lassmann M, Flux G. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1976–1988. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

