Mitochondria mediate septin cage assembly to promote autophagy of Shigella
- PMID: 27259462
- PMCID: PMC4931556
- DOI: 10.15252/embr.201541832
Mitochondria mediate septin cage assembly to promote autophagy of Shigella
Abstract
Septins, cytoskeletal proteins with well-characterised roles in cytokinesis, form cage-like structures around cytosolic Shigella flexneri and promote their targeting to autophagosomes. However, the processes underlying septin cage assembly, and whether they influence S. flexneri proliferation, remain to be established. Using single-cell analysis, we show that the septin cages inhibit S. flexneri proliferation. To study mechanisms of septin cage assembly, we used proteomics and found mitochondrial proteins associate with septins in S. flexneri-infected cells. Strikingly, mitochondria associated with S. flexneri promote septin assembly into cages that entrap bacteria for autophagy. We demonstrate that the cytosolic GTPase dynamin-related protein 1 (Drp1) interacts with septins to enhance mitochondrial fission. To avoid autophagy, actin-polymerising Shigella fragment mitochondria to escape from septin caging. Our results demonstrate a role for mitochondria in anti-Shigella autophagy and uncover a fundamental link between septin assembly and mitochondria.
Keywords: Shigella; autophagy; cytoskeleton; mitochondria; septin.
© 2016 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
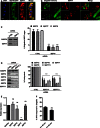
HeLa cells stably expressing SEPT6‐GFP were infected with Shigella flexneri for 4 h 40 min, fixed for confocal microscopy and labelled with antibody for endogenous SEPT7. The scale bar represents 1 μm.
HeLa cells were infected with S. flexneri‐mCherry for 4 h 40 min, fixed for 3D‐SIM and labelled with antibodies to endogenous SEPT7. Inset images show SEPT7 assembly into helix‐like structure around S. flexneri. The scale bar represents 1 μm. See also Movie EV4.
HeLa cells were treated with control (CTRL) or SEPT7 siRNA for 72 h. Whole‐cell lysate of siRNA‐treated cells were immunoblotted for GAPDH or SEPT7 to show the efficiency of SEPT7 depletion. GAPDH was used as a loading control. siRNA‐treated cells were infected with S. flexneri for 4 h 40 min and then fixed and labelled with antibodies to SEPT2, SEPT7 or SEPT9 for quantitative microscopy. Graphs represent the mean % ± SEM of Shigella inside SEPT2, SEPT7 or SEPT9 cages from at least three independent experiments per treatment. Student's t‐test, ***P < 0.001.
HeLa cells were treated with control (CTRL) or two SEPT7 (−1 or −2) siRNA for 72 h, and whole‐cell lysates were immunoblotted for SEPT2, SEPT6, SEPT7, SEPT9 or SEPT11. GAPDH was used as a loading control. Graph represents the mean % ± SEM of the relative amount of protein quantified by densitometry from at least three independent experiments per treatment. Student's t‐test, ***P < 0.001.
HeLa cells were treated with control (CTRL) or SEPT7 siRNA for 72 h, and the transcription level of SEPT2, SEPT6, SEPT7 or SEPT9 was quantified by qRT–PCR. GAPDH was used as control. Graph represents the mean ± SEM of the relative expression of GAPDH, SEPT2, SEPT6, SEPT7 or SEPT9 mRNA from two independent experiments per treatment. Student's t‐test, ns = non‐significant; **P < 0.01.
HeLa cells were infected with x‐light Shigella for 3 h 40 min or 4 h 40 min for quantitative confocal microscopy. IPTG was added 30 min prior to fixation, and then samples were labelled with antibody for SEPT7. Graph represents mean % ± SEM of Shigella responding to IPTG inside SEPT7 cages from at least three independent experiments per time point. Student's t‐test, ns = non‐significant.

Model depicting the x‐light system used for single bacterial analysis. Shigella flexneri M90T carry an inducible plasmid (x‐light Shigella) engineered with the LacI repressor system from Escherichia coli, and a GFP or mCherry gene downstream of the operator. In the presence of IPTG, metabolically active bacteria stop LacI repressor activity. As a consequence, the fluorescent protein is synthesised when bacteria are metabolically active.
HeLa cells were infected with x‐light Shigella‐mCherry for 4 h 40 min for quantitative confocal microscopy. IPTG was added 30 min prior to fixation, and then samples were labelled with antibody for SEPT7. The scale bar represents 5 μm. Graph represents mean % ± SEM of Shigella responding to IPTG outside (−) or inside (+) SEPT7 cages from four independent experiments. Student's t‐test, ***P < 0.001.
HeLa cells were infected with x‐light Shigella‐mCherry for 4 h 40 min for quantitative confocal microscopy. IPTG was added 30 min prior to fixation, and then samples were labelled with antibody for p62. The scale bar represents 5 μm. Graph represents mean % ± SEM of Shigella responding to IPTG without (−) or with (+) p62 from at least four independent experiments. Student's t‐test, ***P < 0.001.
Control (CTRL) or isopropanol‐treated x‐light Shigella‐GFP were induced with IPTG for 30 min and then labelled with SYTOX Orange for 10 min. The scale bar represents 5 μm.
HeLa cells were infected for 2 h 10 min, treated with ethanol (CTRL) or erythromycin (EM) for 2 h prior to fixation and then labelled with antibody for SEPT7. Graph represents mean % ± SEM of Shigella in SEPT7 cages from three independent experiments. Student's t‐test, ***P < 0.001.

Schematic representation of the proteomic experiments used to isolate proteins enriched at the Shigella–septin cage. (a) A SEPT6–STREP–FLAG construct was (b) transfected in HeLa cells for 24 h. (c) Cells were infected with Shigella flexneri AfaI for 4 h, harvested, and (d) SEPT6–STREP–FLAG cage‐associated proteins were isolated through co‐immunoprecipitation (Co‐IP).
HeLa cells stably expressing SEPT6‐GFP were infected with S. flexneri AfaI for 4 h, harvested and then tested with Co‐IP. Empty vector and/or uninfected HeLa cells were used in parallel as control. GFP‐trap magnetic agarose beads were used to isolate SEPT6‐GFP from cells, and extracts were immunoblotted for SEPT6, GFP, SEPT7 or p62. The lysates were immunoblotted for GAPDH as control for cellular protein levels.
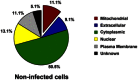
The protein pulldown experiments identified 56 proteins putatively associated with the Shigella–septin cage and then categorised into six groups based on the analysis from the Gene Ontology database. See also Dataset EV1.
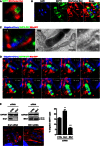
HeLa cells were labelled with MitoTracker Red CMXRos, treated with cytochalasin D for 30 min, then fixed and labelled for endogenous SEPT7 for confocal microscopy. The scale bar represents 0.5 μm. See also Fig EV3A.
HeLa cells were infected with Shigella flexneri for 4 h 40 min, labelled with MitoTracker Red CMXRos, fixed and labelled with antibody for endogenous SEPT7 for quantitative confocal microscopy. The scale bar represents 1 μm.
HeLa cells stably expressing SEPT6‐GFP were transfected with Mito‐BFP for 24 h, infected with Shigella‐mCherry for 4 h 40 min and processed for CLEM. SEPT6 is shown in green, mitochondria in red and Shigella‐mCherry in blue. Septin cages identified by fluorescent light microscopy (FLM) were processed for TEM. The right‐most image is enlarged from the boxed region in the TEM image and shows the bacterium (Sf) surrounded by a double membrane of autophagy (Au) and the mitochondrial membrane (Mt). The scale bar represents 1 μm. See also Fig EV3B.
HeLa cells stably expressing SEPT6‐GFP were transfected with Mito‐BFP for 24 h and then infected with Shigella‐mCherry for 2 h for live confocal microscopy. Time frame sequence shows a septin cage assembling around Shigella. Mitochondria (circle) support the sites of septin ring assembly around the bacterium. Each frame was acquired every 2 min. The scale bar represents 1 μm. See also Movie EV2.
HeLa cells were treated with control (CTRL), Drp1 or Mfn1 siRNA for 72 h. Whole‐cell lysates were immunoblotted for Drp1 or Mfn1 to show the efficiency of depletion. GAPDH was used as a loading control. siRNA‐treated cells were infected with S. flexneri for 4 h 40 min, then fixed for microscopy and stained for endogenous SEPT7 for quantitative confocal microscopy. Graph represents the mean % ± SEM of Shigella inside SEPT7 cages from at least four independent experiments per treatment. Student's t‐test, **P < 0.01; ***P < 0.001.
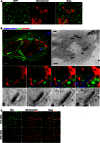
HeLa cells were stained with MitoTracker Red CMXRos, treated with cytochalasin D for 30 min and then fixed and labelled for endogenous SEPT7 for confocal microscopy. Inset images highlight mitochondria closely associated with septin rings. The scale bar represents 5 μm.
HeLa cells stably expressing SEPT6‐GFP were transfected with mito‐BFP for 24 h, infected with Shigella‐mCherry for 4 h 40 min and processed for CLEM. Z‐stack (Z1–Z4) series of septin cages were acquired by fluorescent light microscopy (FLM), and then samples were processed for TEM. SEPT6 is shown in green, mitochondria in red and Shigella in blue. The Z‐stack (Z1–Z4) series clearly shows the septin‐compartmentalised autophagosome and the mitochondrial membrane. The scale bar represents 5 μm.
HeLa cells were treated with control (CTRL) or Mfn1 siRNA for 72 h, labelled with MitoTracker Red CMXRos, fixed for confocal microscopy and labelled with antibodies to Mfn1. The scale bar represents 5 μm. Inset images highlight Mfn1 associated with fused mitochondria fusion in CTRL cells, or absence of Mfn1 associated with fragmented mitochondria in Mfn1‐depleted cells.

HeLa cells expressing SEPT6‐GFP were stained with MitoTracker Red CMXRos and fixed for confocal microscopy. Inset image highlights the location of septins at the sites of mitochondrial fission. The scale bar represents 5 μm.
HeLa cells were fixed and labelled with antibody against SEPT7 and secondary antibody coupled to HRP, and processed for TEM. The right image is enlarged from the boxed region in the TEM image and shows a SEPT7 (S7) filament (labelled in black) intersecting with the mitochondrial (Mt) fission site. The scale bar represents 100 nm. See also Fig EV4.
HeLa cells were stained with MitoTracker Red CMXRos for live SOFI‐based super‐resolution microscopy. Each image was reconstructed from 100 raw TIRF frames using a custom‐made SOFI‐based algorithm. Arrows highlight SEPT6 association with the location of mitochondrial fission. The scale bar represents 1 μm. See also Movie EV4.
HeLa cells were labelled with MitoTracker Red CMXRos, fixed for widefield microscopy and labelled with antibodies to SEPT7 and Drp1. Arrows highlight one example of Drp1 and SEPT7 association with the location of mitochondrial fission. The scale bar represents 1 μm.
HeLa cells were transfected with Mito‐BFP and ActA‐SEPT7 mCherry, fixed and labelled with antibody to Drp1 for widefield microscopy. The scale bar represents 5 μm. Inset images highlight Drp1 and SEPT7 associated with the sites of mitochondrial fission. Pearson's correlation coefficient from three independent experiments (mean ± SEM) shows that the mitochondria in ActA‐SEPT7‐transfected cells recruit significantly more Drp1 than control cells (Mito‐BFP transfected alone). Student's t‐test, *P < 0.05.


HeLa cells were treated with control (CTRL), SEPT7 or Drp1 siRNA for 72 h. Whole‐cell lysate of siRNA‐treated cells were immunoblotted for GAPDH, SEPT7 or Drp1 to show the efficiency of siRNA depletion. GAPDH was used as a loading control. siRNA‐treated cells were labelled with MitoTracker Red CMXRos and fixed for confocal microscopy. The scale bar represents 5 μm.
HeLa cells were treated with control (CTRL), SEPT2, SEPT7, SEPT9, Drp1 or SEPT7 + Drp1 siRNA for 72 h, labelled with MitoTracker Red CMXRos and fixed for quantitative confocal microscopy. Graph represents the length (μm; whiskers from min to max) of mitochondria in siRNA‐treated cells from three independent experiments (analysis of at least 100 measurements per biological replicate). Student's t‐test, ***P < 0.001.
HeLa cells stably expressing SEPT6‐GFP were harvested and then tested with Co‐IP. HeLa cells expressing GFP were used in parallel as control. GFP‐trap magnetic agarose beads were used to isolate SEPT6‐GFP from cells, and extracts were immunoblotted for Drp1, SEPT2, SEPT7 or GFP. The lysates were immunoblotted for GAPDH as control for cellular protein levels.
HeLa cells were treated with control (CTRL) or SEPT7 siRNA for 72 h, and whole‐cell lysates were immunoblotted for SEPT7, Drp1, phosphorylated Drp1 (P‐Drp1) or Mfn1. GAPDH was used as a loading control. Graph represents the mean % ± SEM of the relative amount of Drp1, P‐Drp1, Mfn1 or SEPT7 proteins quantified by densitometry from 4 independent experiments. Student's t‐test, ns: non‐significant; **P < 0.01, ***P < 0.001.
HeLa cells were treated with control (CTRL) or SEPT7 siRNA for 72 h, labelled with MitoTracker Red CMXRos, then fixed and labelled for endogenous P‐Drp1 for quantitative confocal microscopy. Representative images shown here. The scale bar represents 1 μm. The recruitment of P‐Drp1 to mitochondria was significantly decreased as compared to control cells as measured by Pearson's correlation coefficient from at least five independent experiments (mean ± SEM, analysis of at least 250 cells per biological replicate). Student's t‐test, *P < 0.05.
HeLa cells were treated with control (CTRL) or SEPT7 siRNA for 72 h, labelled with MitoTracker Red CMXRos, then fixed and labelled for endogenous Mfn1 for quantitative confocal microscopy. Representative images shown here. The scale bar represents 1 μm. The recruitment of Mfn1 to mitochondria was significantly increased as compared to control cells as measured by Pearson's correlation coefficient from at least five independent experiments (mean ± SEM, analysis of at least 250 cells per biological replicate). Student's t‐test, *P < 0.05.

HeLa cells stably expressing SEPT6‐GFP were treated with Drp1 siRNA for 72 h, transfected with mito‐BFP 24 h and then infected with Shigella flexneri‐mCherry for 2 h for live confocal microscopy. Representative image shows interplay between two Shigella–septin cages and fused mitochondria. The scale bar represents 1 μm. See also Movie EV4.
HeLa cells were treated with Drp1 siRNA for 72 h and then infected with x‐light Shigella‐mCherry for 4 h 40 min for quantitative confocal microscopy. IPTG was added 30 min prior to fixation, and then samples were labelled with antibody for SEPT7. Graph represents mean % ± SEM of Shigella responding to IPTG outside (−) or inside (+) SEPT7 cages from three independent experiments. Student's t‐test, **P < 0.01.
HeLa cells were infected with S. flexneri M90T for 4 h 40 min, labelled with MitoTracker Red CMXRos and fixed for quantitative confocal microscopy. Boxplots show the length of mitochondria (μm; whiskers from min to max) surrounding Shigella outside (−) or inside (+) SEPT7 cages from three independent experiments (analysis of at least 130 measurements per biological replicate). Student's t‐test, ***P < 0.001.
HeLa cells were either kept uninfected as a control or infected with S. flexneri M90T or S. flexneri ΔIcsA for 4 h 40 min. Samples were labelled with MitoTracker Red CMXRos and fixed for quantitative confocal microscopy. Boxplots show length of mitochondria (μm; whiskers from min to max) in uninfected cells (CTRL), surrounding S. flexneri M90T (+M90T) or S. flexneri ΔIcsA (+ΔIcsA) from three independent experiments (analysis of at least 250 measurements per biological replicate). Student's t‐test, ***P < 0.001.
A potential model for the Shigella–septin cage and actin tail assembly pathways. Based on our findings, we propose that (i) mitochondria promote septin cage assembly for antibacterial autophagy or (ii) Shigella fragment mitochondria to counteract septin cage entrapment. Depending on the fragmentation of mitochondria by IcsA, Shigella will be compartmentalised in septin cages or spread cell‐to‐cell via actin‐based motility.
Comment in
-
Priming for destruction: septins at the crossroads of mitochondrial fission and bacterial autophagy.EMBO Rep. 2016 Jul;17(7):935-7. doi: 10.15252/embr.201642606. Epub 2016 Jun 8. EMBO Rep. 2016. PMID: 27279260 Free PMC article.
-
Mitochondria promote septin assembly into cages that entrap Shigella for autophagy.Autophagy. 2018;14(5):913-914. doi: 10.1080/15548627.2016.1228496. Epub 2017 Nov 28. Autophagy. 2018. PMID: 27629779 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

