Loss of AP-2delta reduces retinal ganglion cell numbers and axonal projections to the superior colliculus
- PMID: 27259519
- PMCID: PMC4893287
- DOI: 10.1186/s13041-016-0244-0
Loss of AP-2delta reduces retinal ganglion cell numbers and axonal projections to the superior colliculus
Abstract
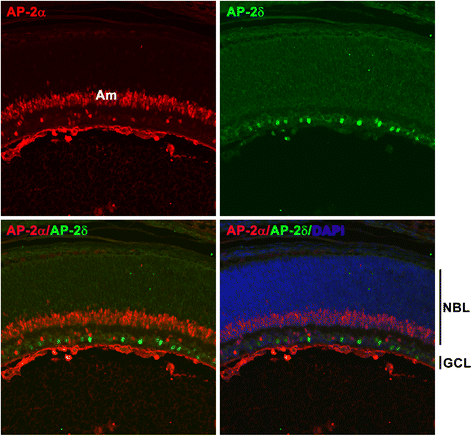
Background: AP-2δ is the most divergent member of the Activating Protein-2 (TFAP2) family of transcription factors. AP-2δ is restricted to specific regions of the CNS, including a subset of ganglion cells in the retina. Retinal ganglion cells (RGCs), the only output neurons of the retina, are responsible for transmitting the visual signal to the brain.
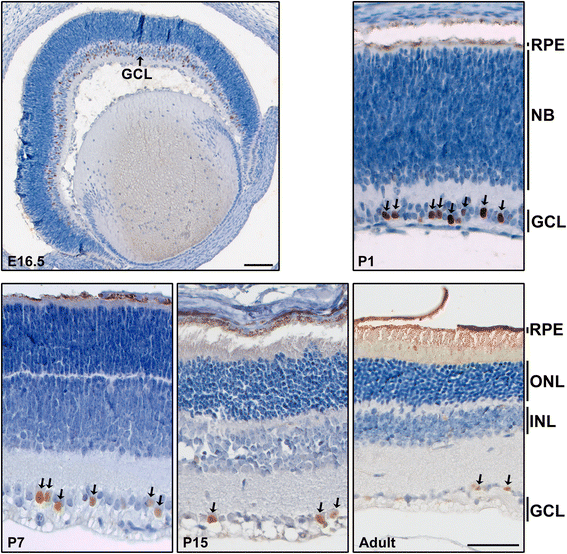
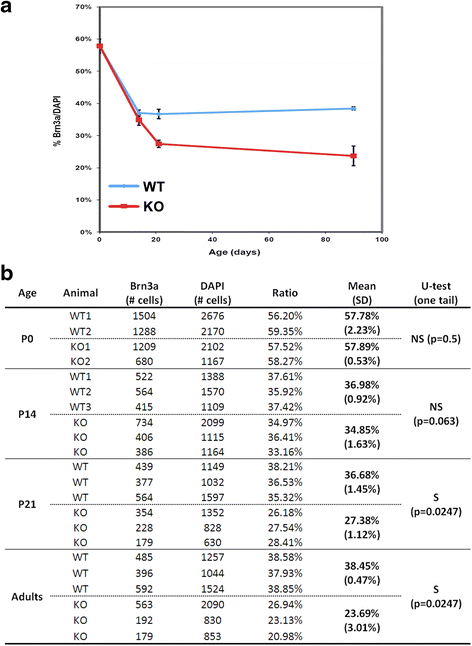
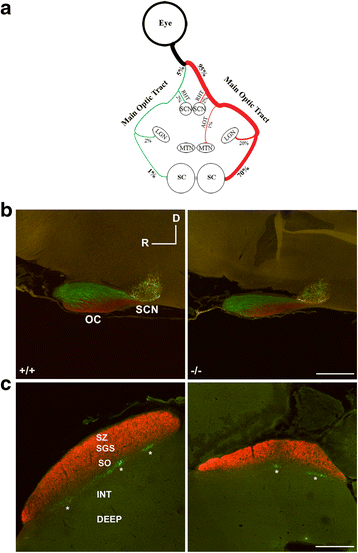
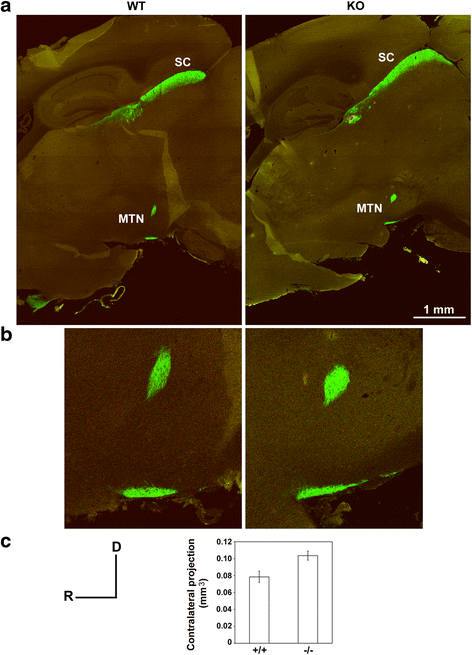
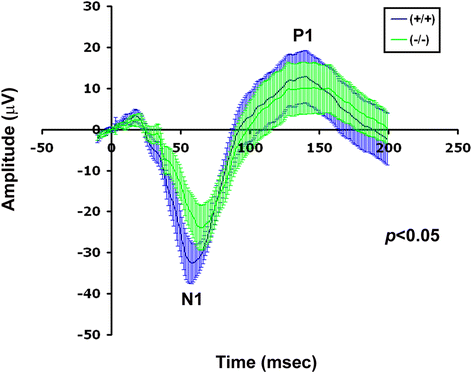
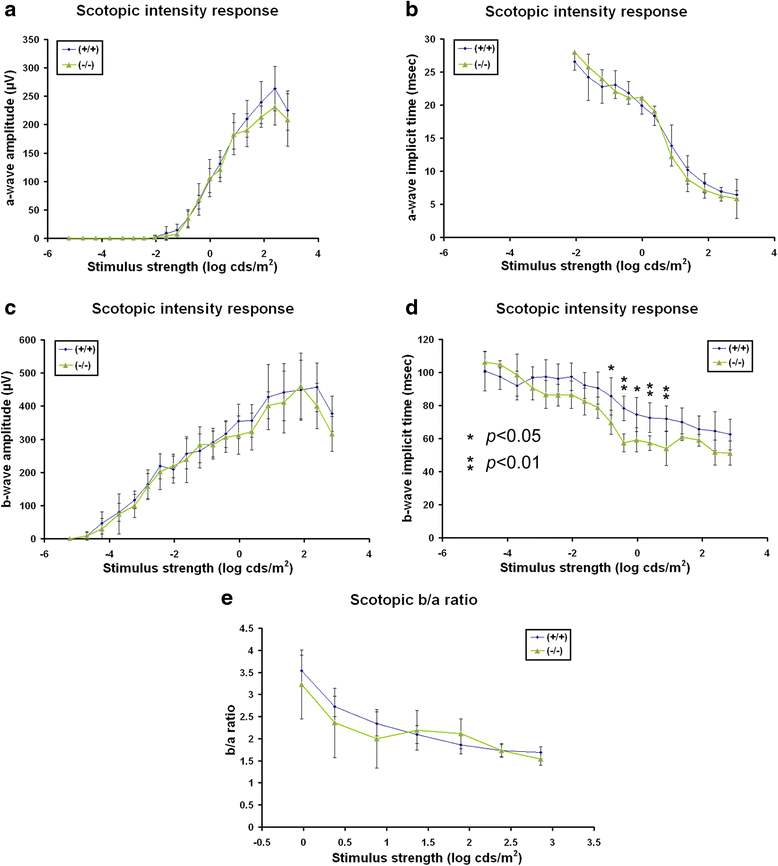
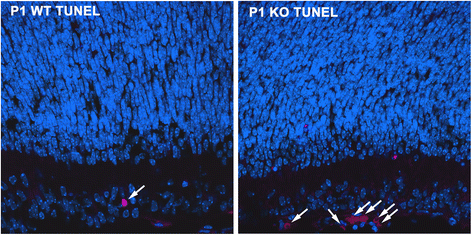
Results: AP-2δ knockout results in loss of Brn3c (Pou4f3) expression in AP-2δ -positive RGCs. While AP-2δ-/- mice have morphologically normal retinas at birth, there is a significant reduction in retinal ganglion cell numbers by P21, after eye opening. Chromatin immunoprecipitation indicates that Brn3c is a target of AP-2δ in the retina. Using fluorochrome-conjugated cholera toxin subunit B to trace ganglion cell axons from the eye to the major visual pathways in the brain, we found 87 % and 32 % decreases in ipsilateral and contralateral projections, respectively, to the superior colliculus in AP-2δ-/- mice. In agreement with anatomical data, visually evoked responses recorded from the brain confirmed that retinal outputs to the brain are compromised.
Conclusions: AP-2δ is important for the maintenance of ganglion cell numbers in the retina. Loss of AP-2δ alters retinal axonal projections to visual centers of the brain, with ipsilaterial projections to the superior colliculus being the most dramatically affected. Our results have important implications for integration of the visual signal at the superior colliculus.
Keywords: AP-2; Axon; Brain; Electrophysiology; Ganglion cells; Retina; Superior colliculus; Transcription factor.
Figures






Similar articles
-
Lack of 5-HT(1B) receptor and of serotonin transporter have different effects on the segregation of retinal axons in the lateral geniculate nucleus compared to the superior colliculus.Neuroscience. 2002;111(3):597-610. doi: 10.1016/s0306-4522(01)00602-9. Neuroscience. 2002. PMID: 12031347
-
Expression of AP-2delta in the developing chick retina.Dev Dyn. 2008 Nov;237(11):3210-21. doi: 10.1002/dvdy.21744. Dev Dyn. 2008. PMID: 18924234
-
Identification of retinal ganglion cell types and brain nuclei expressing the transcription factor Brn3c/Pou4f3 using a Cre recombinase knock-in allele.J Comp Neurol. 2021 Jun;529(8):1926-1953. doi: 10.1002/cne.25065. Epub 2020 Nov 10. J Comp Neurol. 2021. PMID: 33135183 Free PMC article.
-
Spontaneous patterned retinal activity and the refinement of retinal projections.Prog Neurobiol. 2005 Jul;76(4):213-35. doi: 10.1016/j.pneurobio.2005.09.002. Epub 2005 Nov 8. Prog Neurobiol. 2005. PMID: 16280194 Review.
-
Neurotrophins in the developing and regenerating visual system.Histol Histopathol. 1998 Apr;13(2):437-59. doi: 10.14670/HH-13.437. Histol Histopathol. 1998. PMID: 9589902 Review.
Cited by
-
Isolation of Primary Murine Retinal Ganglion Cells (RGCs) by Flow Cytometry.J Vis Exp. 2017 Jul 5;(125):55785. doi: 10.3791/55785. J Vis Exp. 2017. PMID: 28715391 Free PMC article.
-
Identification of multiple transcription factor genes potentially involved in the development of electrosensory versus mechanosensory lateral line organs.Front Cell Dev Biol. 2024 Mar 18;12:1327924. doi: 10.3389/fcell.2024.1327924. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38562141 Free PMC article.
-
Molecular codes for cell type specification in Brn3 retinal ganglion cells.Proc Natl Acad Sci U S A. 2017 May 16;114(20):E3974-E3983. doi: 10.1073/pnas.1618551114. Epub 2017 May 2. Proc Natl Acad Sci U S A. 2017. PMID: 28465430 Free PMC article.
-
Conditional Deletion of AP-2α and AP-2β in the Developing Murine Retina Leads to Altered Amacrine Cell Mosaics and Disrupted Visual Function.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2229-2239. doi: 10.1167/iovs.17-23283. Invest Ophthalmol Vis Sci. 2018. PMID: 29715367 Free PMC article.
-
Genetic control of retinal ganglion cell genesis.Cell Mol Life Sci. 2021 May;78(9):4417-4433. doi: 10.1007/s00018-021-03814-w. Epub 2021 Mar 29. Cell Mol Life Sci. 2021. PMID: 33782712 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

