The subtilisin-like protease SBT3 contributes to insect resistance in tomato
- PMID: 27259555
- PMCID: PMC5301937
- DOI: 10.1093/jxb/erw220
The subtilisin-like protease SBT3 contributes to insect resistance in tomato
Abstract
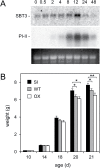
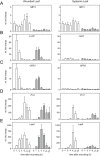
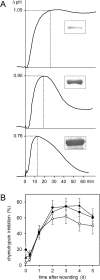
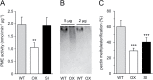
Subtilisin-like proteases (SBTs) constitute a large family of extracellular plant proteases, the function of which is still largely unknown. In tomato plants, the expression of SBT3 was found to be induced in response to wounding and insect attack in injured leaves but not in healthy systemic tissues. The time course of SBT3 induction resembled that of proteinase inhibitor II and other late wound response genes suggesting a role for SBT3 in herbivore defense. Consistent with such a role, larvae of the specialist herbivore Manduca sexta performed better on transgenic plants silenced for SBT3 expression (SBT3-SI). Supporting a contribution of SBT3 to systemic wound signaling, systemic induction of late wound response genes was attenuated in SBT3-SI plants. The partial loss of insect resistance may thus be explained by a reduction in systemic defense gene expression. Alternatively, SBT3 may play a post-ingestive role in plant defense. Similar to other anti-nutritive proteins, SBT3 was found to be stable and active in the insect's digestive system, where it may act on unidentified proteins of insect or plant origin. Finally, a reduction in the level of pectin methylesterification that was observed in transgenic plants with altered levels of SBT3 expression suggested an involvement of SBT3 in the regulation of pectin methylesterases (PMEs). While such a role has been described in other systems, PME activity and the degree of pectin methylesterification did not correlate with the level of insect resistance in SBT3-SI and SBT3 overexpressing plants and are thus unrelated to the observed resistance phenotype.
Keywords: Manduca sexta; pectin methylesterase; proteinase inhibitor; subtilase; systemin; wound signaling..
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures

References
-
- Bédouet L, Courtois B, Courtois J. 2003. Rapid quantification of O-acetyl and O-methyl residues in pectin extracts. Carbohydrate Research 338, 379–383. - PubMed
-
- Calderón-Cortés N, Quesada M, Watanabe H, Cano-Camacho H, Oyama K. 2012. Endogenous plant cell wall digestion: A key mechanism in insect evolution. Annual Review of Ecology, Evolution, and Systematics 43, 45–71.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

