HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer
- PMID: 27259714
- PMCID: PMC4893300
- DOI: 10.1186/s12885-016-2385-z
HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer
Abstract
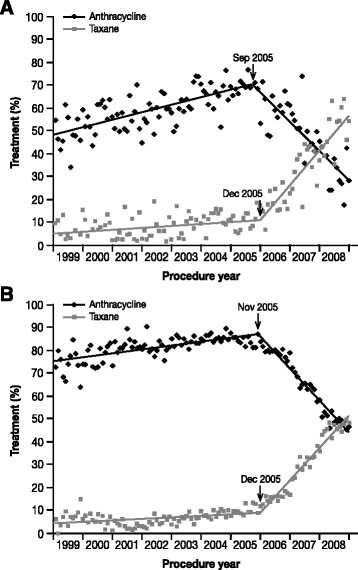
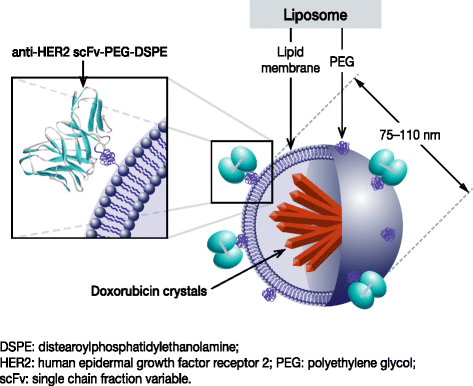
Background: Human epidermal growth factor receptor 2 (HER2)-positive breast cancer is a particularly aggressive form of the disease, and ultimately progresses in patients with metastases on standard therapies. Anthracyclines, such as doxorubicin, are an effective treatment for HER2-positive breast cancer, particularly when administered in combination with trastuzumab - however, doxorubicin-related cardiotoxicity has limited its use. Many patients are therefore never treated with anthracyclines, even upon disease progression, despite the potential for benefit. MM-302 is a novel, HER2-targeted antibody-liposomal doxorubicin conjugate that specifically targets HER2-overexpressing cells. Preclinical and Phase 1 data suggest that MM-302, as a monotherapy or in combination with trastuzumab, could be effective for managing previously treated, anthracycline-naïve, HER2-positive breast cancer, without the cardiotoxicity observed with free doxorubicin formulations.
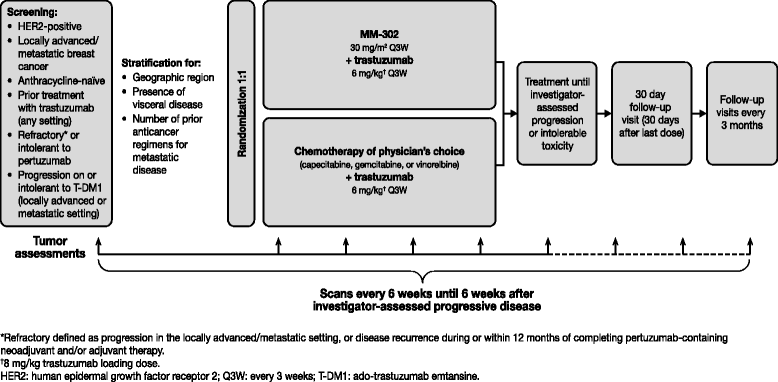
Methods/design: HERMIONE is an open-label, multicenter, randomized (1:1) Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice (gemcitabine, capecitabine, or vinorelbine) plus trastuzumab planned to enroll 250 anthracycline-naïve patients with locally advanced/metastatic HER2-positive breast cancer. Key inclusion criteria are: previous treatment with trastuzumab (with or without pertuzumab) in any setting; refractory or intolerant to pertuzumab (refractory to pertuzumab defined as progression in the locally advanced or metastatic setting, or disease recurrence during or within 12 months of completing pertuzumab-containing neoadjuvant and/or adjuvant therapy); and disease progression on, or intolerant to, ado-trastuzumab emtansine for locally advanced or metastatic disease. The trial is currently being conducted at sites in the USA, Canada, and Western Europe. Treatment will be administered in 21-day cycles, and will be continued until disease progression or unacceptable toxicity. The primary endpoint is independently assessed progression-free survival (PFS). Tumor response will be assessed every 6 weeks, and defined according to RECIST v1.1. Secondary endpoints include investigator-assessed PFS, overall survival (OS), OS rates at 6 months and 1 year, objective response rates, safety and tolerability, quality of life, and the pharmacokinetic profile of MM-302 plus trastuzumab.
Discussion: The HERMIONE study will evaluate the efficacy and safety of MM-302 plus trastuzumab in patients with refractory HER2-positive advanced/metastatic breast cancer for whom there are no standard of care therapies with a proven survival advantage.
Trial registration: Clinicaltrials.gov identifier: NCT02213744 . Registration date: 06AUG2014.
Keywords: Advanced/metastatic breast cancer; Antibody–conjugate; Cardiotoxicity; Doxorubicin; HER2-targeted liposomal doxorubicin; HERMIONE; Human epidermal growth factor receptor 2/HER2/Erb2; Immunoliposome; MM302; Trastuzumab.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

