Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution
- PMID: 27260327
- PMCID: PMC4893272
- DOI: 10.1186/s12934-016-0495-6
Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution
Abstract
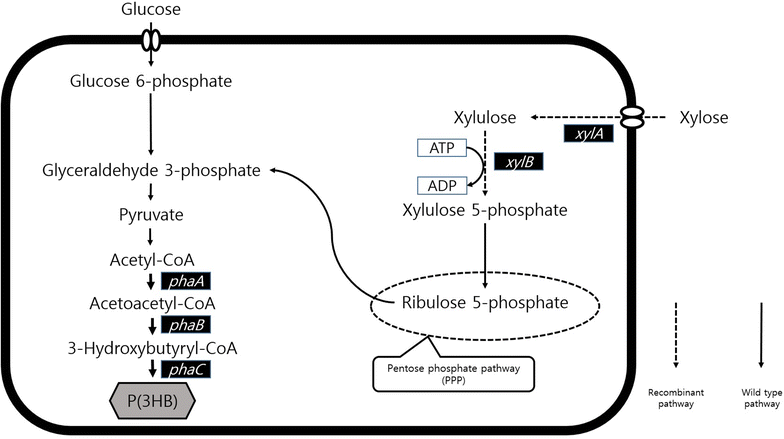
Background: Lignocellulosic raw materials have extensively been examined for the production of bio-based fuels, chemicals, and polymers using microbial platforms. Since xylose is one of the major components of the hydrolyzed lignocelluloses, it is being considered a promising substrate in lignocelluloses based fermentation process. Ralstonia eutropha, one of the most powerful and natural producers of polyhydroxyalkanoates (PHAs), has extensively been examined for the production of bio-based chemicals, fuels, and polymers. However, to the best of our knowledge, lignocellulosic feedstock has not been employed for R. eutropha probably due to its narrow spectrum of substrate utilization. Thus, R. eutropha engineered to utilize xylose should be useful in the development of microbial process for bio-based products from lignocellulosic feedstock.
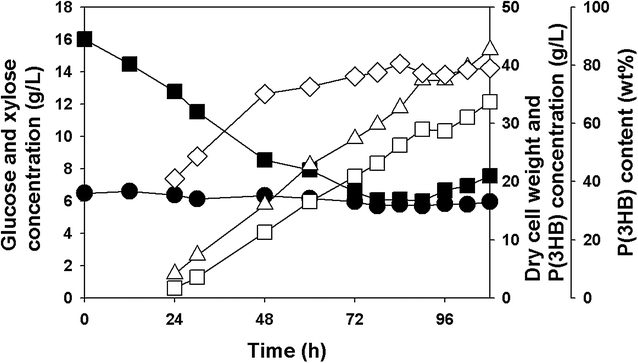
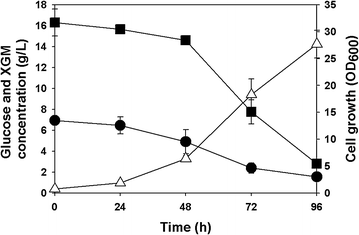
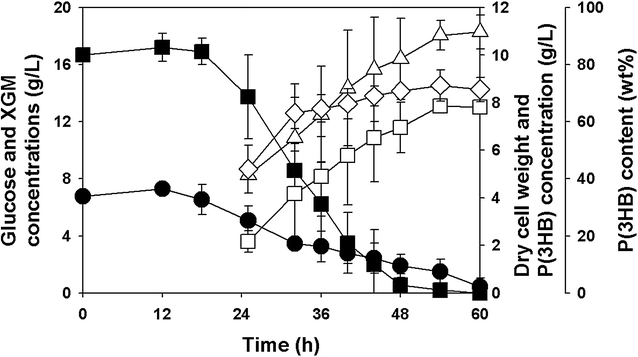
Results: Recombinant R. eutropha NCIMB11599 expressing the E. coli xylAB genes encoding xylose isomerase and xylulokinase respectively, was constructed and examined for the synthesis of poly(3-hydroxybutyrate) [P(3HB)] using xylose as a sole carbon source. It could produce 2.31 g/L of P(3HB) with a P(3HB) content of 30.95 wt% when it was cultured in a nitrogen limited chemically defined medium containing 20.18 g/L of xylose in a batch fermentation. Also, recombinant R. eutropha NCIMB11599 expressing the E. coli xylAB genes produced 5.71 g/L of P(3HB) with a P(3HB) content of 78.11 wt% from a mixture of 10.05 g/L of glucose and 10.91 g/L of xylose in the same culture condition. The P(3HB) concentration and content could be increased to 8.79 g/L and 88.69 wt%, respectively, when it was cultured in the medium containing 16.74 g/L of glucose and 6.15 g/L of xylose. Further examination of recombinant R. eutropha NCIMB11599 expressing the E. coli xylAB genes by fed-batch fermentation resulted in the production of 33.70 g/L of P(3HB) in 108 h with a P(3HB) content of 79.02 wt%. The concentration of xylose could be maintained as high as 6 g/L, which is similar to the initial concentration of xylose during the fed-batch fermentation suggesting that xylose consumption is not inhibited during fermentation. Finally, recombinant R. eutorpha NCIMB11599 expressing the E. coli xylAB gene was examined for the production of P(3HB) from the hydrolysate solution of sunflower stalk. The hydrolysate solution of sunflower stalk was prepared as a model lignocellulosic biomass, which contains 78.8 g/L of glucose, 26.9 g/L of xylose, and small amount of 4.8 g/L of galactose and mannose. When recombinant R. eutropha NCIMB11599 expressing the E. coli xylAB genes was cultured in a nitrogen limited chemically defined medium containing 23.1 g/L of hydrolysate solution of sunflower stalk, which corresponds to 16.8 g/L of glucose and 5.9 g/L of xylose, it completely consumed glucose and xylose in the sunflower stalk based medium resulting in the production of 7.86 g/L of P(3HB) with a P(3HB) content of 72.53 wt%.
Conclusions: Ralstonia eutropha was successfully engineered to utilize xylose as a sole carbon source as well as to co-utilize it in the presence of glucose for the synthesis of P(3HB). In addition, R. eutropha engineered to utilized xylose could synthesize P(3HB) from the sunflower stalk hydrolysate solution containing glucose and xylose as major sugars, which suggests that xylose utilizing R. eutropha developed in this study should be useful for development of lignocellulose based microbial processes.
Keywords: Lignocelluloses; Poly(3-hydroxybutyrate); Ralstonia eutropha; Sunflower stalk; Xylose.
Figures



Similar articles
-
Metabolic engineering of Ralstonia eutropha for the production of polyhydroxyalkanoates from sucrose.Biotechnol Bioeng. 2015 Mar;112(3):638-43. doi: 10.1002/bit.25469. Epub 2014 Oct 21. Biotechnol Bioeng. 2015. PMID: 25258020
-
Biosynthesis of polyhydroxyalkanoates containing 2-hydroxybutyrate from unrelated carbon source by metabolically engineered Escherichia coli.Appl Microbiol Biotechnol. 2012 Jan;93(1):273-83. doi: 10.1007/s00253-011-3530-x. Epub 2011 Aug 14. Appl Microbiol Biotechnol. 2012. PMID: 21842437
-
[Engineering of a D-xylose metabolic pathway in eutropha W50].Wei Sheng Wu Xue Bao. 2014 Jan 4;54(1):42-52. Wei Sheng Wu Xue Bao. 2014. PMID: 24783853 Chinese.
-
Microbial production of poly-D-3-hydroxybutyrate from CO2.Appl Microbiol Biotechnol. 2001 Oct;57(1-2):6-12. doi: 10.1007/s002530100775. Appl Microbiol Biotechnol. 2001. PMID: 11693935 Review.
-
Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review.Int J Biol Macromol. 1999 Jun-Jul;25(1-3):31-6. doi: 10.1016/s0141-8130(99)00012-4. Int J Biol Macromol. 1999. PMID: 10416647 Review.
Cited by
-
Rational engineering of natural polyhydroxyalkanoates producing microorganisms for improved synthesis and recovery.Microb Biotechnol. 2023 Feb;16(2):262-285. doi: 10.1111/1751-7915.14109. Epub 2022 Jul 6. Microb Biotechnol. 2023. PMID: 35792877 Free PMC article. Review.
-
Bio-conversion of organic wastes towards polyhydroxyalkanoates.Biotechnol Notes. 2023 Dec 10;4:118-126. doi: 10.1016/j.biotno.2023.11.006. eCollection 2023. Biotechnol Notes. 2023. PMID: 39416913 Free PMC article. Review.
-
Sugar Beet Molasses as a Potential C-Substrate for PHA Production by Cupriavidus necator.Bioengineering (Basel). 2022 Apr 4;9(4):154. doi: 10.3390/bioengineering9040154. Bioengineering (Basel). 2022. PMID: 35447714 Free PMC article.
-
Recent Advances in the Biosynthesis of Polyhydroxyalkanoates from Lignocellulosic Feedstocks.Life (Basel). 2021 Aug 10;11(8):807. doi: 10.3390/life11080807. Life (Basel). 2021. PMID: 34440551 Free PMC article. Review.
-
xylA and xylB overexpression as a successful strategy for improving xylose utilization and poly-3-hydroxybutyrate production in Burkholderia sacchari.J Ind Microbiol Biotechnol. 2018 Mar;45(3):165-173. doi: 10.1007/s10295-018-2007-7. Epub 2018 Jan 19. J Ind Microbiol Biotechnol. 2018. PMID: 29349569
References
-
- Oh YH, Lee SH, Jang YA, Choi JW, Hong KS, Yu JH, Shin J, Song BK, Mastan SG, David Y, et al. Development of rice bran treatment process and its use for the synthesis of polyhydroxyalkanoates from rice bran hydrolysate solution. Bioresour Technol. 2015;181:283–290. doi: 10.1016/j.biortech.2015.01.075. - DOI - PubMed
-
- Oh YH, Eom IY, Joo JC, Yu JH, Song BK, Lee SH, Hong SH, Park SJ. Recent advances in development of biomass pretreatment technologies used in biorefinery for the production of bio-based fuels, chemicals and polymers. Korean J Chem Eng. 2015;32:1945–1959. doi: 10.1007/s11814-015-0191-y. - DOI
-
- Mamman AS, Lee JM, Kim YC, Hwang IT, Park NJ, Hwang YK, Chang JS, Hwang JS. Furfural: hemicellulose/xylose derived biochemical. Biofuel Bioprod Bior. 2008;2:438–454. doi: 10.1002/bbb.95. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

