Molecular basis for photoreceptor outer segment architecture
- PMID: 27260426
- PMCID: PMC5112118
- DOI: 10.1016/j.preteyeres.2016.05.003
Molecular basis for photoreceptor outer segment architecture
Abstract
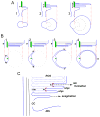
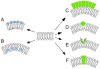
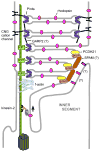
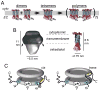
To serve vision, vertebrate rod and cone photoreceptors must detect photons, convert the light stimuli into cellular signals, and then convey the encoded information to downstream neurons. Rods and cones are sensory neurons that each rely on specialized ciliary organelles to detect light. These organelles, called outer segments, possess elaborate architectures that include many hundreds of light-sensitive membranous disks arrayed one atop another in precise register. These stacked disks capture light and initiate the chain of molecular and cellular events that underlie normal vision. Outer segment organization is challenged by an inherently dynamic nature; these organelles are subject to a renewal process that replaces a significant fraction of their disks (up to ∼10%) on a daily basis. In addition, a broad range of environmental and genetic insults can disrupt outer segment morphology to impair photoreceptor function and viability. In this chapter, we survey the major progress that has been made for understanding the molecular basis of outer segment architecture. We also discuss key aspects of organelle lipid and protein composition, and highlight distributions, interactions, and potential structural functions of key OS-resident molecules, including: kinesin-2, actin, RP1, prominin-1, protocadherin 21, peripherin-2/rds, rom-1, glutamic acid-rich proteins, and rhodopsin. Finally, we identify key knowledge gaps and challenges that remain for understanding how normal outer segment architecture is established and maintained.
Keywords: Cilia; Membrane curvature; Outer segment; Photoreceptor; Retinal degeneration; Tetraspanin.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures





References
-
- Abd El-Aziz MM, Barragan I, O’Driscoll CA, Goodstadt L, Prigmore E, Borrego S, Mena M, Pieras JI, El-Ashry MF, Safieh LA, Shah A, Cheetham ME, Carter NP, Chakarova C, Ponting CP, Bhattacharya SS, Antinolo G. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008;40:1285–1287. - PMC - PubMed
-
- Agbaga MM, DK, Brush RS, Lydic T, Conley SM, Naash MI, Gavin RE, Busik JV, Anderson RE. Differential composition of docosahexaenoic acid and very long chain polyunsaturated fatty acids in rod and cone photoreceptor membranes. Invest Ophthalmol Vis Sci. 2014;55:370.
-
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. - PubMed
-
- Anderson RE, Maude MB. Phospholipids of bovine outer segments. Biochem. 1970;9:3624–3628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

