Which outcome measures are reported by clinical trials investigating OME treatment? A case for standardised reporting
- PMID: 27260589
- PMCID: PMC5734610
- DOI: 10.1016/j.ijporl.2016.04.029
Which outcome measures are reported by clinical trials investigating OME treatment? A case for standardised reporting
Abstract
Introduction: Many different OME treatment trials have been published using different outcomes measures to evaluate the success of particular interventions. We set out to identify the variation in reporting of outcome measures in OME trials that exists at present. This has been achieved by reviewing published trials to determine which outcome measures have been reported.
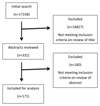
Method: The literature review was carried out using PUBMED database (1980 to 2013). Data were collected on the treatment outcomes reported, with particular focus on the methods of assessment and the number of treatment outcomes used in each study.
Results: The 171 studies identified used 12 broad treatment outcome measures. The most common outcome measure was OME resolution (48%) followed by hearing level (36%). Only 95 studies used a single outcome measure, with 76 studies using between 2 and 4 outcome measures. The method of assessment varied between studies that used the same treatment outcome measures.
Conclusion: OME treatment trials report a wide range of measures and comparison across studies is thus difficult. Establishing a core set of outcome measures to be reported by all trials in the future could be useful, and would allow comprehensive comparison of different studies and minimise potential for reporting bias.
Keywords: Clinical trial; Otitis media; Outcomes.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures
References
-
- Fried VM, Mukuc DM, Rooks RN. Ambulatory health care visits by children: principle diagnosis and places of visit. Vital Health Statistics. 1998;13:1–23. - PubMed
-
- Gates GA. Cost-effectiveness considerations in otitis media treatment. Otolaryngology-Head and Neck surgery. 1996;114:525–530. - PubMed
-
- Bondy J, Berman S, Glazner J, et al. Direct expenditures related to otitis media diagnosis: extrapolations from a pediatric Medicaid cohort. Pediatrics. 2000;105:e72. - PubMed
-
- NICE. Surgical Management of Children with Otitis Media with Effusion (OME) London: National Institute for Health and Clinical Excellence; 2008.
-
- Rosenfeld RM, Kay D. Natural history of untreated otitis media with effusion. Laryngoscope. 2003;113(10):1645–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical