Mutations in Dnaaf1 and Lrrc48 Cause Hydrocephalus, Laterality Defects, and Sinusitis in Mice
- PMID: 27261005
- PMCID: PMC4978901
- DOI: 10.1534/g3.116.030791
Mutations in Dnaaf1 and Lrrc48 Cause Hydrocephalus, Laterality Defects, and Sinusitis in Mice
Abstract
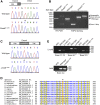
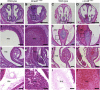
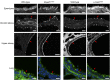
We have previously described a forward genetic screen in mice for abnormalities of brain development. Characterization of two hydrocephalus mutants by whole-exome sequencing after whole-genome SNP mapping revealed novel recessive mutations in Dnaaf1 and Lrrc48 Mouse mutants of these two genes have not been previously reported. The Dnaaf1 mutant carries a mutation at the splice donor site of exon 4, which results in abnormal transcripts. The Lrrc48 mutation is a missense mutation at a highly conserved leucine residue, which is also associated with a decrease in Lrrc48 transcription. Both Dnaaf1 and Lrrc48 belong to a leucine-rich repeat-containing protein family and are components of the ciliary axoneme. Their Chlamydomonas orthologs are known to be required for normal ciliary beat frequency or flagellar waveform, respectively. Some Dnaaf1 or Lrrc48 homozygote mutants displayed laterality defects, suggesting a motile cilia defect in the embryonic node. Mucus accumulation and neutrophil infiltration in the maxillary sinuses suggested sinusitis. Dnaaf1 mutants showed postnatal lethality, and none survived to weaning age. Lrrc48 mutants survive to adulthood, but had male infertility. ARL13B immunostaining showed the presence of motile cilia in the mutants, and the distal distribution of DNAH9 in the axoneme of upper airway motile cilia appeared normal. The phenotypic abnormalities suggest that mutations in Dnaaf1 and Lrrc48 cause defects in motile cilia function.
Keywords: Dnaaf1/Lrrc50/Oda7; ENU mutagenesis; Lrrc48/FAP134/Drc3; motile cilia; primary ciliary dyskinesia.
Copyright © 2016 Ha et al.
Figures



Similar articles
-
DNAAF1 links heart laterality with the AAA+ ATPase RUVBL1 and ciliary intraflagellar transport.Hum Mol Genet. 2018 Feb 1;27(3):529-545. doi: 10.1093/hmg/ddx422. Hum Mol Genet. 2018. PMID: 29228333 Free PMC article.
-
Mutations in DNAJB13, Encoding an HSP40 Family Member, Cause Primary Ciliary Dyskinesia and Male Infertility.Am J Hum Genet. 2016 Aug 4;99(2):489-500. doi: 10.1016/j.ajhg.2016.06.022. Am J Hum Genet. 2016. PMID: 27486783 Free PMC article.
-
Mutations in the Motile Cilia Gene DNAAF1 Are Associated with Neural Tube Defects in Humans.G3 (Bethesda). 2016 Oct 13;6(10):3307-3316. doi: 10.1534/g3.116.033696. G3 (Bethesda). 2016. PMID: 27543293 Free PMC article.
-
Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia.J Neurosci Res. 2013 Sep;91(9):1117-32. doi: 10.1002/jnr.23238. Epub 2013 May 17. J Neurosci Res. 2013. PMID: 23686703 Review.
-
Mammalian axoneme central pair complex proteins: Broader roles revealed by gene knockout phenotypes.Cytoskeleton (Hoboken). 2016 Jan;73(1):3-22. doi: 10.1002/cm.21271. Cytoskeleton (Hoboken). 2016. PMID: 26785425 Free PMC article. Review.
Cited by
-
PCD Genes-From Patients to Model Organisms and Back to Humans.Int J Mol Sci. 2022 Feb 3;23(3):1749. doi: 10.3390/ijms23031749. Int J Mol Sci. 2022. PMID: 35163666 Free PMC article. Review.
-
Congenital hydrocephalus: new Mendelian mutations and evidence for oligogenic inheritance.Hum Genomics. 2023 Mar 2;17(1):16. doi: 10.1186/s40246-023-00464-w. Hum Genomics. 2023. PMID: 36859317 Free PMC article.
-
The role of a ciliary GTPase in the regulation of neuronal maturation of olfactory sensory neurons.Development. 2023 Jan 15;150(2):dev201116. doi: 10.1242/dev.201116. Epub 2023 Jan 19. Development. 2023. PMID: 36661357 Free PMC article.
-
The regulatory roles of motile cilia in CSF circulation and hydrocephalus.Fluids Barriers CNS. 2021 Jul 7;18(1):31. doi: 10.1186/s12987-021-00265-0. Fluids Barriers CNS. 2021. PMID: 34233705 Free PMC article. Review.
-
The RNA splicing factor PRPF8 is required for left-right organiser cilia function and determination of cardiac left-right asymmetry via regulation of Arl13b splicing.bioRxiv [Preprint]. 2025 May 27:2025.05.22.654869. doi: 10.1101/2025.05.22.654869. bioRxiv. 2025. PMID: 40501629 Free PMC article. Preprint.
References
-
- Badano J. L., Mitsuma N., Beales P. L., Katsanis N., 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7: 125–148. - PubMed
-
- Banizs B., Pike M. M., Millican C. L., Ferguson W. B., Komlosi P., et al. , 2005. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132: 5329–5339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
