Relationships Between RNA Polymerase II Activity and Spt Elongation Factors to Spt- Phenotype and Growth in Saccharomyces cerevisiae
- PMID: 27261007
- PMCID: PMC4978902
- DOI: 10.1534/g3.116.030346
Relationships Between RNA Polymerase II Activity and Spt Elongation Factors to Spt- Phenotype and Growth in Saccharomyces cerevisiae
Abstract
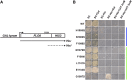
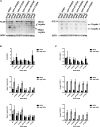
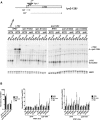
The interplay between adjacent transcription units can result in transcription-dependent alterations in chromatin structure or recruitment of factors that determine transcription outcomes, including the generation of intragenic or other cryptic transcripts derived from cryptic promoters. Mutations in a number of genes in Saccharomyces cerevisiae confer both cryptic intragenic transcription and the Suppressor of Ty (Spt(-)) phenotype for the lys2-128∂ allele of the LYS2 gene. Mutants that suppress lys2-128∂ allow transcription from a normally inactive Ty1 ∂ promoter, conferring a LYS(+) phenotype. The arrangement of transcription units at lys2-128∂ is reminiscent of genes containing cryptic promoters within their open reading frames. We set out to examine the relationship between RNA Polymerase II (Pol II) activity, functions of Spt elongation factors, and cryptic transcription because of the previous observation that increased-activity Pol II alleles confer an Spt(-) phenotype. We identify both cooperating and antagonistic genetic interactions between Pol II alleles and alleles of elongation factors SPT4, SPT5, and SPT6 We find that cryptic transcription at FLO8 and STE11 is distinct from that at lys2-128∂, though all show sensitivity to reduction in Pol II activity, especially the expression of lys2-128∂ found in Spt(-) mutants. We determine that the lys2-128∂ Spt(-) phenotypes for spt6-1004 and increased activity rpo21/rpb1 alleles each require transcription from the LYS2 promoter. Furthermore, we identify the Ty1 transcription start site (TSS) within the ∂ element as the position of Spt(-) transcription in tested Spt(-) mutants.
Keywords: Ty1 element; cryptic transcription; gene expression; transcription elongation; transcription initiation.
Copyright © 2016 Cui et al.
Figures









References
-
- Amberg D. C., Burke D. J., Strathern J. N., 2005. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2005 Edition Cold Spring Harbor Press, Cold Spring Harbor, NY.
-
- Boeke J. D., Trueheart J., Natsoulis G., Fink G. R., 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
