Determinants of hepatotoxicity after repeated supratherapeutic paracetamol ingestion: systematic review of reported cases
- PMID: 27261770
- PMCID: PMC5137817
- DOI: 10.1111/bcp.13028
Determinants of hepatotoxicity after repeated supratherapeutic paracetamol ingestion: systematic review of reported cases
Abstract
Aims: To evaluate the role of reported daily dose, age and other risk factors, and to assess the value of quantifying serum transaminase activity and paracetamol (acetaminophen) concentration at initial assessment for identifying patients at risk of hepatotoxicity following repeated supratherapeutic paracetamol ingestion (RSPI).
Methods: Systematic literature review with collation and analysis of individual-level data from reported cases of RSPI associated with liver damage.
Results: In 199 cases meeting the selection criteria, severe liver damage (ALT/AST ≥1000 IU l(-1) , liver failure or death) was reported in 186 (93%) cases including 77/78 (99%) children aged ≤6 years. Liver failure occurred in 127 (64%) cases; of these 49 (39%) died. Maximum ingested daily paracetamol doses were above UK recommendations in 143 (72%) patients. US-Australasian thresholds for repeated supratherapeutic ingestions requiring intervention were not met in 71 (36%) cases; of these 35 (49%) developed liver failure and 10 (14%) died. No cases developing liver damage had paracetamol concentration < 20 mg l(-1) and a normal ALT/AST on initial presentation or when RSPI was first suspected, but both of these values were only available for 79 (40%) cases.
Conclusions: Severe liver damage is reported after RSPI in adults and children, sometimes involving reported doses below current thresholds for intervention. Paracetamol concentrations <20 mg l(-1) with normal serum ALT/AST activity on initial assessment suggests a low risk of subsequent liver damage. These findings are, however, limited by low patient numbers, publication bias and the accuracy of the histories in reported cases.
Keywords: acetaminophen hepatotoxicity; drug induced liver damage; liver injury; paracetamol hepatotoxicity; repeated supratherapeutic acetaminophen ingestion; repeated supratherapeutic paracetamol ingestion.
© 2016 The British Pharmacological Society.
Figures
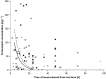
 ) and US (− −) treatment lines for acute paracetamol ingestion
) and US (− −) treatment lines for acute paracetamol ingestion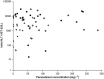
Similar articles
-
Risk prediction of hepatotoxicity in paracetamol poisoning.Clin Toxicol (Phila). 2017 Sep;55(8):879-892. doi: 10.1080/15563650.2017.1317349. Epub 2017 Apr 27. Clin Toxicol (Phila). 2017. PMID: 28447858
-
Interventions for paracetamol (acetaminophen) overdose.Cochrane Database Syst Rev. 2018 Feb 23;2(2):CD003328. doi: 10.1002/14651858.CD003328.pub3. Cochrane Database Syst Rev. 2018. PMID: 29473717 Free PMC article.
-
Paracetamol (acetaminophen) poisoning; consensus definitions of poisoning types and outcomes to be used in the clinical toxicology recommendations collaborative systematic review.Clin Toxicol (Phila). 2025 May;63(5):343-347. doi: 10.1080/15563650.2025.2479721. Epub 2025 Mar 31. Clin Toxicol (Phila). 2025. PMID: 40162912
-
Tramadol with or without paracetamol (acetaminophen) for cancer pain.Cochrane Database Syst Rev. 2017 May 16;5(5):CD012508. doi: 10.1002/14651858.CD012508.pub2. Cochrane Database Syst Rev. 2017. PMID: 28510996 Free PMC article.
-
Paracetamol (acetaminophen) for acute treatment of episodic tension-type headache in adults.Cochrane Database Syst Rev. 2016 Jun 16;2016(6):CD011889. doi: 10.1002/14651858.CD011889.pub2. Cochrane Database Syst Rev. 2016. PMID: 27306653 Free PMC article.
Cited by
-
Suspected paracetamol overdose in Monrovia, Liberia: a matched case-control study.BMC Pediatr. 2020 Mar 30;20(1):139. doi: 10.1186/s12887-020-2008-3. BMC Pediatr. 2020. PMID: 32228536 Free PMC article.
-
Analysis of common causes of liver damage among children 12 years and younger in Weifang.J Int Med Res. 2021 Apr;49(4):3000605211006661. doi: 10.1177/03000605211006661. J Int Med Res. 2021. PMID: 33827321 Free PMC article.
-
Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults.Medicina (Kaunas). 2021 Jul 25;57(8):752. doi: 10.3390/medicina57080752. Medicina (Kaunas). 2021. PMID: 34440958 Free PMC article.
References
-
- Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over‐the‐counter medication use among US preschool‐age children. JAMA 1994; 272: 1025–30. - PubMed
-
- Lesko SM, Mitchell AA. An assessment of the safety of pediatric ibuprofen: a practitioner‐based randomized clinical trial. JAMA 1995; 273: 929–33. - PubMed
-
- Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy 2007; 27: 1219–30. - PubMed
-
- Whyte IM, Francis B, Dawson AH. Safety and efficacy of intravenous N‐acetylcysteine for acetaminophen overdose: analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin 2007; 23: 2359–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

