Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer
- PMID: 27262168
- PMCID: PMC5206761
- DOI: 10.1001/jamaoncol.2016.1828
Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer
Erratum in
-
Incorrect Affiliations and Updated Supplement.JAMA Oncol. 2016 Nov 1;2(11):1511. doi: 10.1001/jamaoncol.2016.4680. JAMA Oncol. 2016. PMID: 27685939 No abstract available.
Abstract
Importance: A critical decision in the management of metastatic castration-resistant prostate cancer (mCRPC) is when to administer an androgen receptor signaling (ARS) inhibitor or a taxane.
Objective: To determine if pretherapy nuclear androgen-receptor splice variant 7 (AR-V7) protein expression and localization on circulating tumor cells (CTCs) is a treatment-specific marker for response and outcomes between ARS inhibitors and taxanes.
Design, setting, and participants: For this cross-sectional cohort study at Memorial Sloan Kettering Cancer Center, 265 men with progressive mCRPC undergoing a change in treatment were considered; 86 were excluded because they were not initiating ARS or taxane therapy; and 18 were excluded for processing time constraints, leaving 161 patients for analysis. Between December 2012 and March 2015, blood was collected and processed from patients with progressive mCRPC immediately prior to new line of systemic therapy. Patients were followed up to 3 years.
Main outcomes and measures: Prostate-specific antigen (PSA) response, time receiving therapy, radiographic progression-free survival (rPFS), and overall survival (OS).
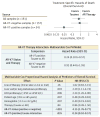
Results: Overall, of 193 prospectively collected blood samples from 161 men with mCRPC, 191 were evaluable (128 pre-ARS inhibitor and 63 pretaxane). AR-V7-positive CTCs were found in 34 samples (18%), including 3% of first-line, 18% of second-line, and 31% of third- or greater line samples. Patients whose samples had AR-V7-positive CTCs before ARS inhibition had resistant posttherapy PSA changes (PTPC), shorter rPFS, shorter time on therapy, and shorter OS than those without AR-V7-positive CTCs. Overall, resistant PTPC were seen in 65 of 112 samples (58%) without detectable AR-V7-positive CTCs prior to ARS inhibition. There were statistically significant differences in OS but not in PTPC, time on therapy, or rPFS for patients with or without pretherapy AR-V7-positive CTCs treated with a taxane. A multivariable model adjusting for baseline factors associated with survival showed superior OS with taxanes relative to ARS inhibitors when AR-V7-positive CTCs were detected pretherapy (hazard ratio, 0.24; 95% CI, 0.10-0.57; P = .035).
Conclusions and relevance: The results validate CTC nuclear expression of AR-V7 protein in men with mCRPC as a treatment-specific biomarker that is associated with superior survival on taxane therapy over ARS-directed therapy in a clinical practice setting. Continued examination of this biomarker in prospective studies will further aid clinical utility.
Conflict of interest statement
Disclosures: Dr Scher reports nonfinancial support from Janssen and Medivation, personal fees from Astellas and Sanofi Aventis, and research funding from Janssen Diagnostics, Janssen Pharmaceuticals, and Medivation. Dr Danila reports financial support from Janssen and Medivation-Astellas. Drs Lu, Graf, Greene, Marrinucci; Ms Louw, Ms Johnson; and Mr Jendrisak, Mr Wahl, and Mr Dittamore are employees of Epic Sciences. No other disclosures are reported.
Figures



Comment in
-
AR-V7 Protein in Circulating Tumor Cells-The Decider for Therapy?JAMA Oncol. 2016 Nov 1;2(11):1450-1451. doi: 10.1001/jamaoncol.2016.2360. JAMA Oncol. 2016. PMID: 27261766 No abstract available.
-
Re: Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker with Outcomes and Survival in Castration-Resistant Prostate Cancer.J Urol. 2016 Oct;196(4):1124. doi: 10.1016/j.juro.2016.07.066. Epub 2016 Jul 18. J Urol. 2016. PMID: 27628794 No abstract available.
References
-
- Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24(12):2972–2977. - PubMed
-
- Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65(1):30–36. - PubMed
-
- Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11(3):129–144. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

