DNA Polymerases Divide the Labor of Genome Replication
- PMID: 27262731
- PMCID: PMC4993630
- DOI: 10.1016/j.tcb.2016.04.012
DNA Polymerases Divide the Labor of Genome Replication
Abstract
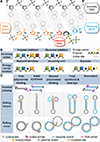
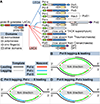
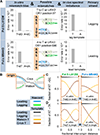
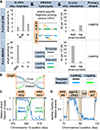
DNA polymerases synthesize DNA in only one direction, but large genomes require RNA priming and bidirectional replication from internal origins. We review here the physical, chemical, and evolutionary constraints underlying these requirements. We then consider the roles of the major eukaryotic replicases, DNA polymerases α, δ, and ɛ, in replicating the nuclear genome. Pol α has long been known to extend RNA primers at origins and on Okazaki fragments that give rise to the nascent lagging strand. Taken together, more recent results of mutation and ribonucleotide incorporation mapping, electron microscopy, and immunoprecipitation of nascent DNA now lead to a model wherein Pol ɛ and Pol δ, respectively, synthesize the majority of the nascent leading and lagging strands of undamaged DNA.
Keywords: DNA replication; evolution; genomic ribonucleotide; polymerase; replication fork.
Published by Elsevier Ltd.
Figures


References
-
- Kornberg A, Baker TA. DNA replication. W.H. Freeman; 1992.
-
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. - PubMed
-
- Kainuma-Kuroda R, Okazaki R. Mechanism of DNA chain growth. XII. Asymmetry of replication of P2 phage DNA. J Mol Biol. 1975;94:213–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

