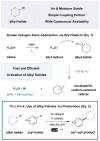
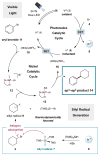
Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling
- PMID: 27263662
- PMCID: PMC5103281
- DOI: 10.1021/jacs.6b04818
Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling
Abstract
A strategy for cross-electrophile coupling has been developed via the merger of photoredox and transition metal catalysis. In this report, we demonstrate the use of commercially available tris(trimethylsilyl)silane with metallaphotoredox catalysis to efficiently couple alkyl bromides with aryl or heteroaryl bromides in excellent yields. We hypothesize that a photocatalytically generated silyl radical species can perform halogen-atom abstraction to activate alkyl halides as nucleophilic cross-coupling partners. This protocol allows the use of mild yet robust conditions to construct Csp(3)-Csp(2) bonds generically via a unique cross-coupling pathway.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
-
For some reviews of the use of nickel in cross-coupling methods, see: Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299.Netherton MR, Fu GC. Adv Synth Catal. 2004;346:1525.Rudolph A, Lautens M. Angew Chem, Int Ed. 2009;48:2656.
-
-
- Knappke CEI, Grupe S, Gärtner D, Corpet M, Gosmini C, Jacobi von Wangelin A. Chem - Eur J. 2014;20:6828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources