The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites
- PMID: 27264604
- PMCID: PMC5026192
- DOI: 10.1016/j.cell.2016.05.018
The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites
Erratum in
-
The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites.Cell. 2016 Nov 3;167(4):1137. doi: 10.1016/j.cell.2016.10.034. Cell. 2016. PMID: 27814510 No abstract available.
Abstract
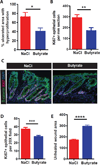
In the mammalian intestine, crypts of Leiberkühn house intestinal epithelial stem/progenitor cells at their base. The mammalian intestine also harbors a diverse array of microbial metabolite compounds that potentially modulate stem/progenitor cell activity. Unbiased screening identified butyrate, a prominent bacterial metabolite, as a potent inhibitor of intestinal stem/progenitor proliferation at physiologic concentrations. During homeostasis, differentiated colonocytes metabolized butyrate likely preventing it from reaching proliferating epithelial stem/progenitor cells within the crypt. Exposure of stem/progenitor cells in vivo to butyrate through either mucosal injury or application to a naturally crypt-less host organism led to inhibition of proliferation and delayed wound repair. The mechanism of butyrate action depended on the transcription factor Foxo3. Our findings indicate that mammalian crypt architecture protects stem/progenitor cell proliferation in part through a metabolic barrier formed by differentiated colonocytes that consume butyrate and stimulate future studies on the interplay of host anatomy and microbiome metabolism.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Authors with no conflict of interest.
Figures






Comment in
-
Colonic Crypts: Safe Haven from Microbial Products.Cell. 2016 Jun 16;165(7):1564-1566. doi: 10.1016/j.cell.2016.06.003. Cell. 2016. PMID: 27315471
-
Host response: Keeping microbial metabolites at bay.Nat Rev Microbiol. 2016 Aug;14(8):474-5. doi: 10.1038/nrmicro.2016.95. Epub 2016 Jun 20. Nat Rev Microbiol. 2016. PMID: 27320504 No abstract available.
-
Colonic crypts are natural gatekeepers of microbial metabolites to protect stem cells.Transl Cancer Res. 2016 Sep;5(Suppl 3):S536-S539. doi: 10.21037/tcr.2016.08.24. Transl Cancer Res. 2016. PMID: 30906700 Free PMC article. No abstract available.
References
-
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Current opinion in cell biology. 2006;18:185–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

