Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo
- PMID: 27264607
- PMCID: PMC5606228
- DOI: 10.1016/j.cell.2016.05.031
Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo
Abstract
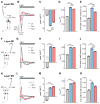
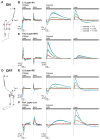
A mechanistic understanding of neural computation requires determining how information is processed as it passes through neurons and across synapses. However, it has been challenging to measure membrane potential changes in axons and dendrites in vivo. We use in vivo, two-photon imaging of novel genetically encoded voltage indicators, as well as calcium imaging, to measure sensory stimulus-evoked signals in the Drosophila visual system with subcellular resolution. Across synapses, we find major transformations in the kinetics, amplitude, and sign of voltage responses to light. We also describe distinct relationships between voltage and calcium signals in different neuronal compartments, a substrate for local computation. Finally, we demonstrate that ON and OFF selectivity, a key feature of visual processing across species, emerges through the transformation of membrane potential into intracellular calcium concentration. By imaging voltage and calcium signals to map information flow with subcellular resolution, we illuminate where and how critical computations arise.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Neuronal Computations Made Visible with Subcellular Resolution.Cell. 2016 Jun 30;166(1):18-20. doi: 10.1016/j.cell.2016.06.022. Cell. 2016. PMID: 27368098
References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. - PubMed
-
- Borst A. Fly visual course control: behaviour, algorithms and circuits. Nat Rev Neurosci. 2014;15:590–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

