Novel MRI Contrast Agent from Magnetotactic Bacteria Enables In Vivo Tracking of iPSC-derived Cardiomyocytes
- PMID: 27264636
- PMCID: PMC4893600
- DOI: 10.1038/srep26960
Novel MRI Contrast Agent from Magnetotactic Bacteria Enables In Vivo Tracking of iPSC-derived Cardiomyocytes
Abstract
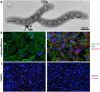
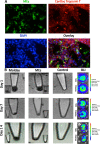
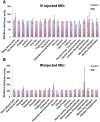
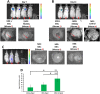
Therapeutic delivery of human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (iCMs) represents a novel clinical approach to regenerate the injured myocardium. However, methods for robust and accurate in vivo monitoring of the iCMs are still lacking. Although superparamagnetic iron oxide nanoparticles (SPIOs) are recognized as a promising tool for in vivo tracking of stem cells using magnetic resonance imaging (MRI), their signal persists in the heart even weeks after the disappearance of the injected cells. This limitation highlights the inability of SPIOs to distinguish stem cell viability. In order to overcome this shortcoming, we demonstrate the use of a living contrast agent, magneto-endosymbionts (MEs) derived from magnetotactic bacteria for the labeling of iCMs. The ME-labeled iCMs were injected into the infarcted area of murine heart and probed by MRI and bioluminescence imaging (BLI). Our findings demonstrate that the MEs are robust and effective biological contrast agents to track iCMs in an in vivo murine model. We show that the MEs clear within one week of cell death whereas the SPIOs remain over 2 weeks after cell death. These findings will accelerate the clinical translation of in vivo MRI monitoring of transplanted stem cell at high spatial resolution and sensitivity.
Figures
References
-
- Urbanek K. et al.. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circulation research 97, 663–673 (2005). - PubMed
-
- Amir G. et al.. Dynamics of human myocardial progenitor cell populations in the neonatal period. The Annals of thoracic surgery 86, 1311–1319 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

