Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid
- PMID: 27264717
- PMCID: PMC4931959
- DOI: 10.1016/j.ejpb.2016.05.023
Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid
Abstract
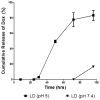
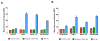
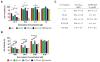
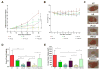
Cancer-specific drug delivery represents an attractive approach to prevent undesirable side-effects and increase the accumulation of the drug in the tumor. Surface modification of nanoparticles such as liposomes with targeting moieties specific to the up-regulated receptors on the surface of tumor cells thus represents an effective strategy. Furthermore, since this receptor expression can be heterogeneous, using a dual-combination of targeting moieties may prove advantageous. With this in mind, the anti-cancer activity of PEGylated doxorubicin-loaded liposomes targeted with folic acid (F), transferrin (Tf) or both (F+Tf) was evaluated. The dual-targeted liposomes showed a 7-fold increase in cell association compared to either of the single-ligand targeted ones in human cervical carcinoma (HeLa) cell monolayers. The increased penetration and cell association of the dual-targeted liposomes were also demonstrated using HeLa cell spheroids. The in vitro cytotoxicity of the doxorubicin liposomes (LD) was then evaluated using HeLa and A2780-ADR ovarian carcinoma cell monolayers. In both these cell lines, the (F+Tf) LD showed significantly higher cytotoxic effects than the untargeted, or single-ligand targeted liposomes. In a HeLa xenograft model in nude mice, compared to the untreated group, though the untargeted LD showed 42% tumor growth inhibition, both the (F) LD and (F+Tf) LD showed 75% and 79% tumor growth inhibition respectively. These results thus highlight that though the dual-targeted liposomes represent an effective cytotoxic formulation in the in vitro setting, they were equally effective as the folic acid-targeted liposomes in reducing tumor burden in the more complex in vivo setting in this particular model.
Keywords: A2780-ADR; Cancer; Doxorubicin; Dual-targeting; Folic acid; HeLa; Liposomes; Nanomedicine; Nanoparticles; Receptor targeting; Transferrin; Xenograft.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, Henley SJ, Eheman CR, Anderson RN, Penberthy L. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):djv048. - PMC - PubMed
-
- Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473–80. - PubMed
-
- Bhattacharjee H, Balabathula P, Wood GC. Targeted nanoparticulate drug-delivery systems for treatment of solid tumors a review. Ther Deliv. 2010;1(5):713–34. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. - PubMed
-
- Bellarosa D, Ciucci A, Bullo A, Nardelli F, Manzini S, Maggi CA, Goso C. Apoptotic events in a human ovarian cancer cell line exposed to anthracyclines. J Pharmacol Exp Ther. 2001;296(2):276–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

