The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells
- PMID: 27264969
- PMCID: PMC4893612
- DOI: 10.1038/srep26979
The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells
Erratum in
-
Corrigendum: The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells.Sci Rep. 2016 Jul 29;6:30667. doi: 10.1038/srep30667. Sci Rep. 2016. PMID: 27472448 Free PMC article. No abstract available.
Abstract
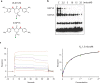
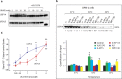
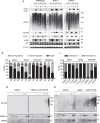
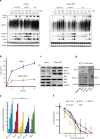
Inhibition of deubiquitinase (DUB) activity is a promising strategy for cancer therapy. VLX1570 is an inhibitor of proteasome DUB activity currently in clinical trials for relapsed multiple myeloma. Here we show that VLX1570 binds to and inhibits the activity of ubiquitin-specific protease-14 (USP14) in vitro, with comparatively weaker inhibitory activity towards UCHL5 (ubiquitin-C-terminal hydrolase-5). Exposure of multiple myeloma cells to VLX1570 resulted in thermostabilization of USP14 at therapeutically relevant concentrations. Transient knockdown of USP14 or UCHL5 expression by electroporation of siRNA reduced the viability of multiple myeloma cells. Treatment of multiple myeloma cells with VLX1570 induced the accumulation of proteasome-bound high molecular weight polyubiquitin conjugates and an apoptotic response. Sensitivity to VLX1570 was moderately affected by altered drug uptake, but was unaffected by overexpression of BCL2-family proteins or inhibitors of caspase activity. Finally, treatment with VLX1570 was found to lead to extended survival in xenograft models of multiple myeloma. Our findings demonstrate promising antiproliferative activity of VLX1570 in multiple myeloma, primarily associated with inhibition of USP14 activity.
Conflict of interest statement
J.G., P.D. and S.L. are shareholders in Vivolux AB.
Figures


Similar articles
-
Sensitivity of Acute Myelocytic Leukemia Cells to the Dienone Compound VLX1570 Is Associated with Inhibition of the Ubiquitin-Proteasome System.Biomolecules. 2021 Sep 10;11(9):1339. doi: 10.3390/biom11091339. Biomolecules. 2021. PMID: 34572552 Free PMC article.
-
Acute lymphoblastic leukemia cells are sensitive to disturbances in protein homeostasis induced by proteasome deubiquitinase inhibition.Oncotarget. 2017 Mar 28;8(13):21115-21127. doi: 10.18632/oncotarget.15501. Oncotarget. 2017. PMID: 28423502 Free PMC article.
-
Analysis of determinants for in vitro resistance to the small molecule deubiquitinase inhibitor b-AP15.PLoS One. 2019 Oct 22;14(10):e0223807. doi: 10.1371/journal.pone.0223807. eCollection 2019. PLoS One. 2019. PMID: 31639138 Free PMC article.
-
Proteasome inhibitors for multiple myeloma.Jpn J Clin Oncol. 2018 Sep 1;48(9):785-793. doi: 10.1093/jjco/hyy108. Jpn J Clin Oncol. 2018. PMID: 30102324 Review.
-
Proteasome inhibitors in the clinical setting: benefits and strategies to overcome multiple myeloma resistance to proteasome inhibitors.Drugs R D. 2007;8(1):1-12. doi: 10.2165/00126839-200708010-00001. Drugs R D. 2007. PMID: 17249845 Review.
Cited by
-
Advances in Deubiquitinating Enzyme Inhibition and Applications in Cancer Therapeutics.Cancers (Basel). 2020 Jun 15;12(6):1579. doi: 10.3390/cancers12061579. Cancers (Basel). 2020. PMID: 32549302 Free PMC article. Review.
-
USP14 Regulates DNA Damage Response and Is a Target for Radiosensitization in Non-Small Cell Lung Cancer.Int J Mol Sci. 2020 Sep 2;21(17):6383. doi: 10.3390/ijms21176383. Int J Mol Sci. 2020. PMID: 32887472 Free PMC article.
-
Repurposing old drugs as new inhibitors of the ubiquitin-proteasome pathway for cancer treatment.Semin Cancer Biol. 2021 Jan;68:105-122. doi: 10.1016/j.semcancer.2019.12.013. Epub 2019 Dec 26. Semin Cancer Biol. 2021. PMID: 31883910 Free PMC article. Review.
-
The emerging role of deubiquitylating enzymes as therapeutic targets in cancer metabolism.Cancer Cell Int. 2022 Mar 20;22(1):130. doi: 10.1186/s12935-022-02524-y. Cancer Cell Int. 2022. PMID: 35307036 Free PMC article. Review.
-
Regulation of Wnt Signaling through Ubiquitination and Deubiquitination in Cancers.Int J Mol Sci. 2020 May 30;21(11):3904. doi: 10.3390/ijms21113904. Int J Mol Sci. 2020. PMID: 32486158 Free PMC article. Review.
References
-
- Hershko A. & Ciechanover A. The ubiquitin system. Ann Rev Biochem 67, 425–479 (1998). - PubMed
-
- Groll M. et al. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 386, 463–471 (1997). - PubMed
-
- Goldberg A. L. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans 35, 12–17 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

