Membrane remodelling in bacteria
- PMID: 27265614
- PMCID: PMC6168058
- DOI: 10.1016/j.jsb.2016.05.010
Membrane remodelling in bacteria
Abstract
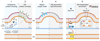
In bacteria the ability to remodel membrane underpins basic cell processes such as growth, and more sophisticated adaptations like inter-cell crosstalk, organelle specialisation, and pathogenesis. Here, selected examples of membrane remodelling in bacteria are presented and the diverse mechanisms for inducing membrane fission, fusion, and curvature discussed. Compared to eukaryotes, relatively few curvature-inducing proteins have been characterised so far. Whilst it is likely that many such proteins remain to be discovered, it also reflects the importance of alternative membrane remodelling strategies in bacteria where passive mechanisms for generating curvature are utilised.
Keywords: Bacteria; Curvature-inducing protein; Membrane; Remodelling; Vesicle.
Copyright © 2016. Published by Elsevier Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

