Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction
- PMID: 27265864
- PMCID: PMC4967372
- DOI: 10.1016/j.ydbio.2016.05.035
Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction
Abstract
Both Fras1 and Itga8 connect mesenchymal cells to epithelia by way of an extracellular 'Fraser protein complex' that functions in signaling and adhesion; these proteins are vital to the development of several vertebrate organs. We previously found that zebrafish fras1 mutants have craniofacial defects, specifically, shortened symplectic cartilages and cartilage fusions that spare joint elements. During a forward mutagenesis screen, we identified a new zebrafish mutation, b1161, that we show here disrupts itga8, as confirmed using CRISPR-generated itga8 alleles. fras1 and itga8 single mutants and double mutants have similar craniofacial phenotypes, a result expected if loss of either gene disrupts function of the Fraser protein complex. Unlike fras1 mutants or other Fraser-related mutants, itga8 mutants do not show blistered tail fins. Thus, the function of the Fraser complex differs in the craniofacial skeleton and the tail fin. Focusing on the face, we find that itga8 mutants consistently show defective outpocketing of a late-forming portion of the first pharyngeal pouch, and variably express skeletal defects, matching previously characterized fras1 mutant phenotypes. In itga8 and fras1 mutants, skeletal severity varies markedly between sides, indicating that both mutants have increased developmental instability. Whereas fras1 is expressed in epithelia, we show that itga8 is expressed complementarily in facial mesenchyme. Paired with the observed phenotypic similarity, this expression indicates that the genes function in epithelial-mesenchymal interactions. Similar interactions between Fras1 and Itga8 have previously been found in mouse kidney, where these genes both regulate Nephronectin (Npnt) protein abundance. We find that zebrafish facial tissues express both npnt and the Fraser gene fibrillin2b (fbn2b), but their transcript levels do not depend on fras1 or itga8 function. Using a revertible fras1 allele, we find that the critical window for fras1 function in the craniofacial skeleton is between 1.5 and 3 days post fertilization, which coincides with the onset of fras1-dependent and itga8-dependent morphogenesis. We propose a model wherein Fras1 and Itga8 interact during late pharyngeal pouch morphogenesis to sculpt pharyngeal arches through epithelial-mesenchymal interactions, thereby stabilizing the developing craniofacial skeleton.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
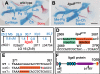


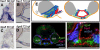
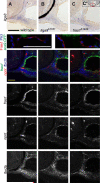



References
-
- Beattie CE, Raible DW, Henion PD, Eisen JS. Early pressure screens. Methods Cell Biol. 1999;60:71–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

