Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment
- PMID: 27266704
- PMCID: PMC4896021
- DOI: 10.1186/s12943-016-0528-0
Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment
Abstract
Background: The 3-dimensional (3D) microenvironment of breast carcinomas is characterized by profoundly altered pH homeostasis, reflecting increased metabolic acid production and a confined extracellular space characterized by poor diffusion, yet the relative contributions of specific pH-regulatory transporters to 3D growth are poorly understood. The aim of this work was to determine how 3D spheroid growth of breast cancer cells impacts the expression and spatial organization of major acid extruding proteins, and how these proteins in turn are required for spheroid growth.
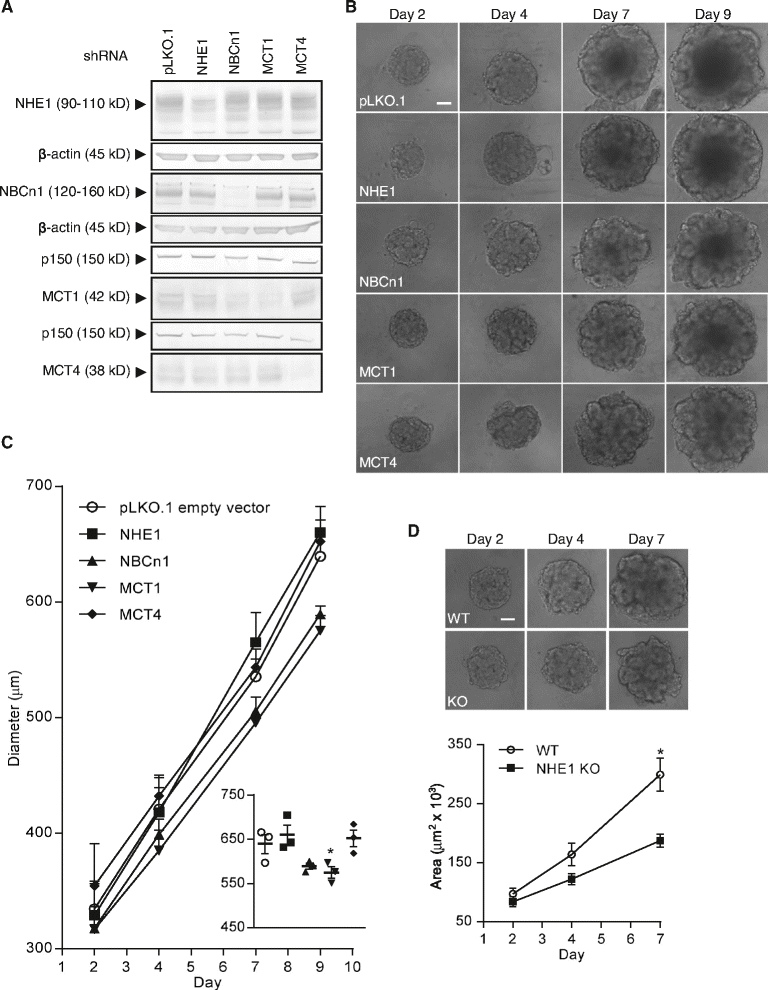
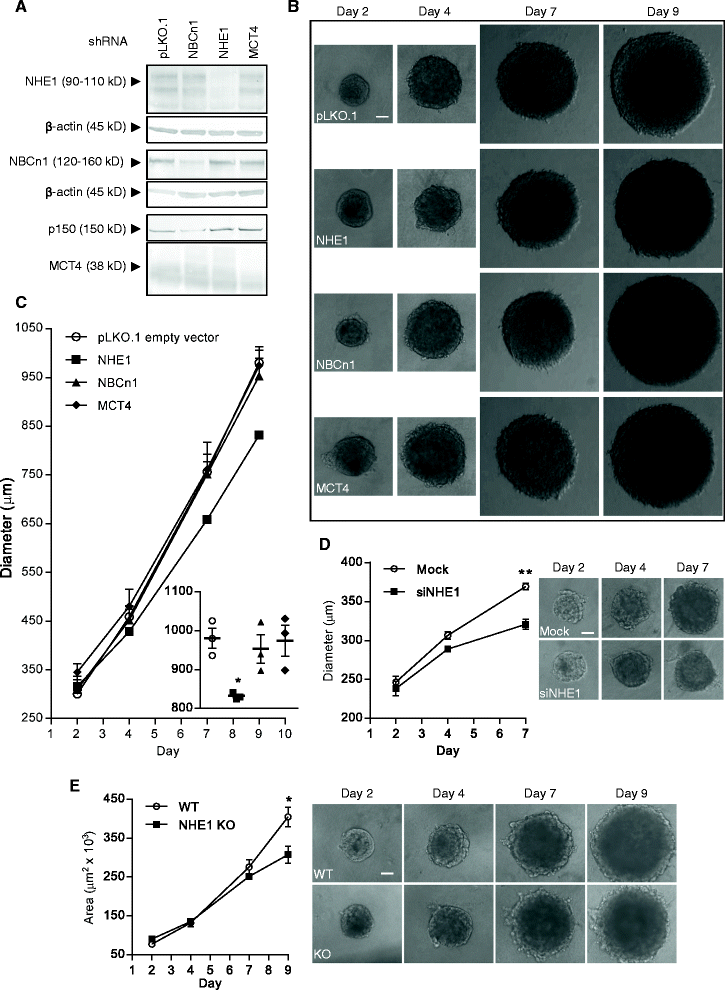
Methods: MCF-7 (Luminal-A) and MDA-MB-231 (Triple-negative) human breast cancer cells were grown as ~700-950 μm diameter spheroids, which were subjected to Western blotting for relevant transporters (2- and 3D growth), quantitative immunohistochemical analysis, and spheroid growth assays. Individual transporter contributions were assessed (i) pharmacologically, (ii) by stable shRNA- and transient siRNA-mediated knockdown, and (iii) by CRISPR/Cas9 knockout.
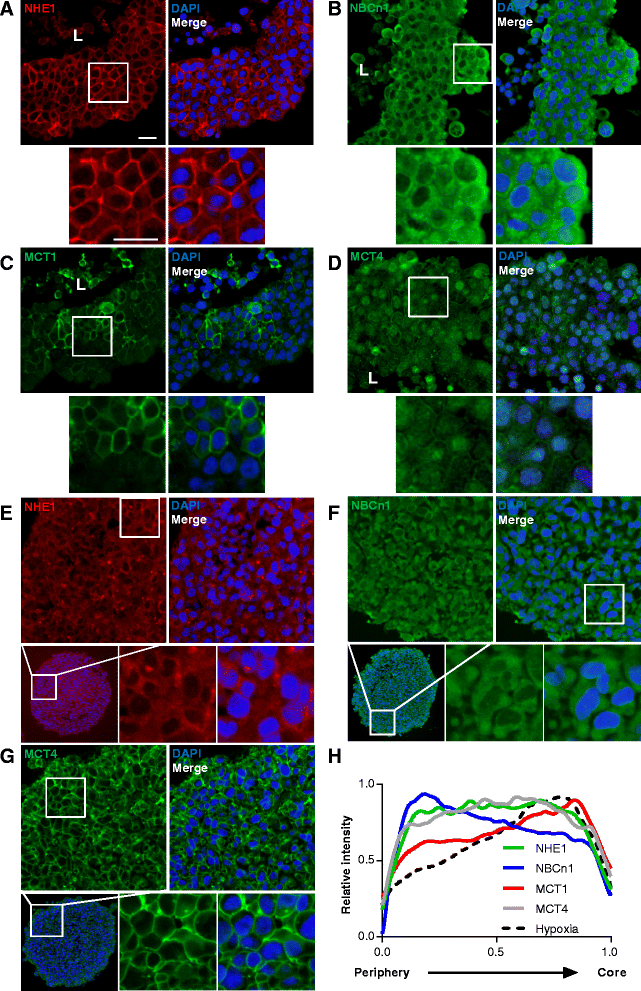
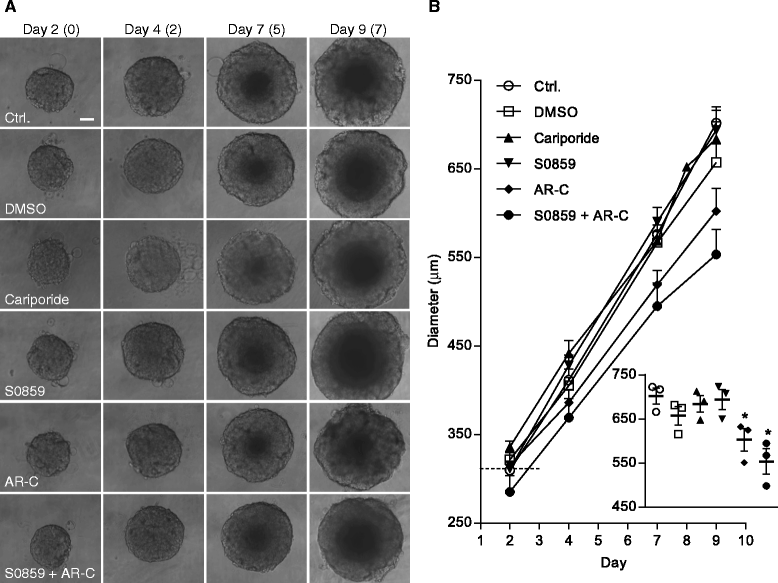
Results: In MCF-7 spheroids, expression of the lactate-H(+) cotransporter MCT1 (SLC16A1) increased from the spheroid periphery to its core, the Na(+),HCO3 (-) cotransporter NBCn1 (SLC4A7) was most highly expressed at the periphery, and the Na(+)/H(+) exchanger NHE1 (SLC9A1) and MCT4 (SLC16A3) were evenly distributed. A similar pattern was seen in MDA-MB-231 spheroids, except that these cells do not express MCT1. The relative total expression of NBCn1 and NHE1 was decreased in 3D compared to 2D, while that of MCT1 and MCT4 was unaltered. Inhibition of MCT1 (AR-C155858) attenuated MCF-7 spheroid growth and this was exacerbated by addition of S0859, an inhibitor of Na(+),HCO3 (-) cotransporters and MCTs. The pharmacological data was recapitulated by stable knockdown of MCT1 or NBCn1, whereas knockdown of MCT4 had no effect. CRISPR/Cas9 knockout of NHE1, but neither partial NHE1 knockdown nor the NHE1 inhibitor cariporide, inhibited MCF-7 spheroid growth. In contrast, growth of MDA-MB-231 spheroids was inhibited by stable or transient NHE1 knockdown and by NHE1 knockout, but not by knockdown of NBCn1 or MCT4.
Conclusions: This work demonstrates the distinct expression and localization patterns of four major acid-extruding transporters in 3D spheroids of human breast cancer cells and reveals that 3D growth is dependent on these transporters in a cell type-dependent manner, with potentially important implications for breast cancer therapy.
Keywords: Acid–base transport; MCT1; MCT4; NBCn1; NHE1; Tumor microenvironment.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

