Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation
- PMID: 27266957
- PMCID: PMC4968910
- DOI: 10.1080/15384101.2016.1191706
Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation
Abstract
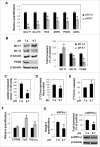
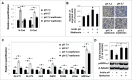
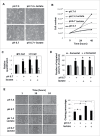
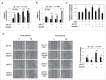
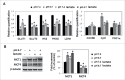
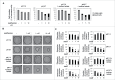
Low extracellular pH promotes in melanoma cells a malignant phenotype characterized by an epithelial-to-mesenchymal transition (EMT) program, endowed with mesenchymal markers, high invasiveness and pro-metastatic property. Here, we demonstrate that melanoma cells exposed to an acidic extracellular microenvironment, 6.7±0.1, shift to an oxidative phosphorylation (Oxphos) metabolism. Metformin, a biguanide commonly used for type 2 diabetes, inhibited the most relevant features of acid-induced phenotype, including EMT and Oxphos. When we tested effects of lactic acidosis, to verify whether sodium lactate might have additional effects on acidic melanoma cells, we found that EMT and Oxphos also characterized lactic acid-treated cells. An increased level of motility was the only gained property of lactic acidic-exposed melanoma cells. Metformin treatment inhibited both EMT markers and Oxphos and, when its concentration raised to 10 mM, it induced a striking inhibition of proliferation and colony formation of acidic melanoma cells, both grown in protons enriched medium or lactic acidosis. Thus, our study provides the first evidence that metformin may target either proton or lactic acidosis-exposed melanoma cells inhibiting EMT and Oxphox metabolism. These findings disclose a new potential rationale of metformin addition to advanced melanoma therapy, e.g. targeting acidic cell subpopulation.
Keywords: Acidic microenvironment; cell metabolism; lactic acidosis; melanoma cells; metformin.
Figures

References
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4:891-9; PMID:15516961; http://dx.doi.org/10.1038/nrc1478 - DOI - PubMed
-
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029-33; PMID:19460998; http://dx.doi.org/10.1126/science.1160809 - DOI - PMC - PubMed
-
- Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, et al.. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med 2006; 47:885-95; PMID:16644760 - PubMed
-
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8:705-713; PMID:19143055; http://dx.doi.org/10.1038/nrc2468 - DOI - PubMed
-
- Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S, Garcìa AG, Harguindey S, Fais S. Proton channels and exchangers in cancer. Biochim Biophys Acta 2015; 1848:2715-26; PMID:25449995; http://dx.doi.org/10.1016/j.bbamem.2014.10.015 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
