Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis
- PMID: 27267134
- PMCID: PMC4895880
- DOI: 10.1186/s12887-016-0607-9
Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis
Abstract
Background: Many clinicians have concerns about the safety of atopic dermatitis (AD) treatments, particularly in children requiring long-term daily maintenance therapy. Topical corticosteroids (TCS) have been widely used for >5 decades. Long-term TCS monotherapy has been associated with adverse cutaneous effects including atrophy, rebound flares, and increased percutaneous absorption with potential for adverse systemic effects. Topical calcineurin inhibitors (TCIs), tacrolimus and pimecrolimus, available for 1-2 decades, are not associated with atrophy or increased percutaneous absorption after prolonged use and have much lower potential for systemic effects. However, since 2006 TCIs have carried a controversial Boxed Warning based on a theoretical risk of malignancy (eg, skin and lymphoma) that has limited TCI use for standard-of-care maintenance therapy.
Methods: A comparative systematic search of PubMed was done for long-term (≥12 week) clinical trials of TCS or TCI treatment in patients <12 years with AD. Citations were reviewed for inclusion based on MeSH terms, abstracts, and relevant article text. Studies were excluded if they did not encompass subjects <12 years, or were <12 weeks' duration, retrospective, meta-analyses, or limited to anecdotal case reports.
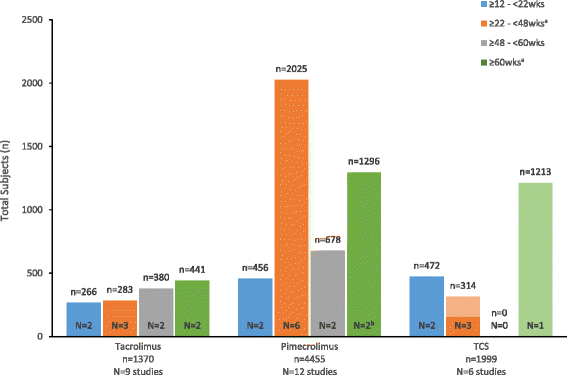
Results: Of 27 trials meeting criteria, 21 included 5825 pediatric patients treated with TCIs, and 6 included 1999 patients treated with TCS. TCS studies were limited to low- to mid-potency products, and all but one study lacked a vehicle control. Eight TCI studies were vehicle-controlled, and safety data were well reported, with ≤5 % of patients reporting discontinuation due to adverse effects (DAEs). Cutaneous and systemic adverse events (AEs) were similar in TCI and vehicle groups, with no reports of lymphoma. Safety data in TCS trials were less well reported. DAE incidence was addressed in just 2 trials, and systemic and cutaneous AEs were mostly unreported.
Conclusions: Data supporting long-term use of TCIs are robust, documenting safety and efficacy, while data supporting long-term TCS use are limited to low- to mid-potency products. Our review identifies a lack of information on the safety of commonly prescribed, long-term monotherapy with mid- to high-potency TCS in pediatric AD, and supports standard-of-care maintenance therapy with TCIs and intermittent use of low- to mid-potency TCS for flares.
Keywords: Atopic dermatitis; Long-term safety; Lymphoma; Pediatric; Pimecrolimus; TCI; TCS; Tacrolimus; Topical calcineurin inhibitor; Topical corticosteroid.
Figures


References
-
- Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. Hyattsville: National Center for Health Statistics; 2013. - PubMed
-
- Eichenfield LF, Boguniewicz M, Simpson EL, Russell JJ, Block JK, Feldman SR et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015. [In Progress] - PubMed
-
- Saavedra JM, Boguniewicz M, Chamlin S, Lake A, Nedorost S, Czerkies LA, et al. Patterns of clinical management of atopic dermatitis in infants and toddlers: a survey of three physician specialties in the United States. J Pediatr. 2013;163(6):1747–1753. doi: 10.1016/j.jpeds.2013.06.073. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

