Questions regarding the safety and duration of immunity following live yellow fever vaccination
- PMID: 27267203
- PMCID: PMC5171234
- DOI: 10.1080/14760584.2016.1198259
Questions regarding the safety and duration of immunity following live yellow fever vaccination
Abstract
The World Health Organization (WHO) and other health agencies have concluded that yellow fever booster vaccination is unnecessary since a single dose of vaccine confers lifelong immunity. Areas covered: We reviewed the clinical studies cited by health authorities in their investigation of both the safety profile and duration of immunity for the YFV-17D vaccine and examined the position that booster vaccination is no longer needed. We found that antiviral immunity may be lost in 1-in-3 to 1-in-5 individuals within 5 to 10 years after a single vaccination and that children may be at greater risk for primary vaccine failure. The safety profile of YFV-17D was compared to other licensed vaccines including oral polio vaccine (OPV) and the rotavirus vaccine, RotaShield, which have subsequently been withdrawn from the US and world market, respectively. Expert commentary: Based on these results and recent epidemiological data on vaccine failures (particularly evident at >10 years after vaccination), we believe that current recommendations to no longer administer YFV-17D booster vaccination be carefully re-evaluated, and that further development of safer vaccine approaches should be considered.
Keywords: Yellow fever virus; antibody duration; flaviviruses; immunity; neurotropic; vaccination; viscerotropic.
Conflict of interest statement
Declaration of InterestsThis project was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases R44 AI079898 (to MK Slifka and IJ Amanna), R01 AI098723 (to MK Slifka) and Oregon National Primate Research Center grant, 8P51 OD011092-53 (to MK Slifka). OHSU, MK Slifka, and IJ Amanna have a financial interest in Najít Technologies, Inc., a company that is developing a new yellow fever vaccine based on a hydrogen peroxide-based inactivation approach. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures



References
-
- Russell MN, Cetron MS, Eidex RB. The US-Certified Yellow Fever Vaccination Center Registry: a tool for travelers, state health departments, and vaccine providers. J Travel Med. 2006;13(1):48–49. - PubMed
-
- Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–173. - PubMed
-
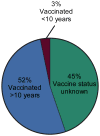
- Camara FP, de Carvalho LM, Gomes AL. Demographic profile of sylvatic yellow fever in Brazil from 1973 to 2008. Trans R Soc Trop Med Hyg. 2013;107(5):324–327. Describes 831 cases of yellow fever in Brazil with 459 (55%) of the patients described as having a history of YFV-17D vaccination. This indicates that YFV-17D vaccine failures are more common than previously realized. - PubMed
-
- Camacho LA, da Freire MS, da Leal ML, et al. Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004;38(5):671–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
