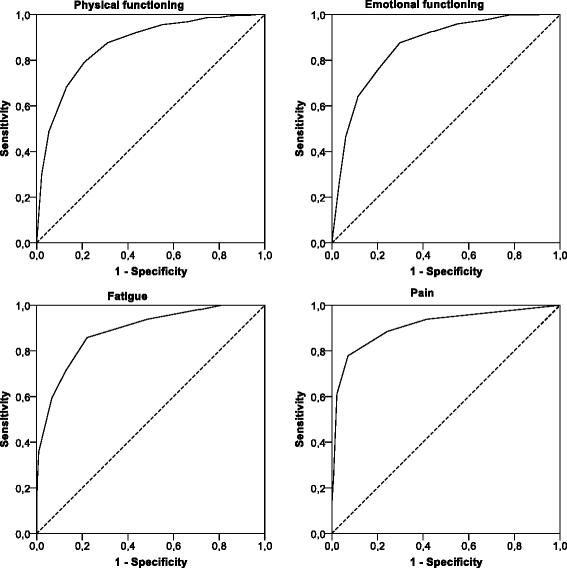
Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain
- PMID: 27267486
- PMCID: PMC4897949
- DOI: 10.1186/s12955-016-0489-4
Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain
Abstract
Background: The EORTC QLQ-C30 is one of the most widely used quality of life questionnaires in cancer research. Availability of thresholds for clinical importance for the individual questionnaire domains could help to increase its interpretability. The aim of our study was to identify thresholds for clinical importance for four EORTC QLQ-C30 scales: Physical Functioning (PF), Emotional Functioning (EF), Pain (PA) and Fatigue (FA).
Methods: We recruited adult cancer patients from Austria, the Netherlands, Poland and the UK. No restrictions were placed on diagnosis or type or stage of treatment. Patients completed the QLQ-C30 and three anchor items reflecting potential attributes of clinically important levels of PF, EF, PA and FA. We merged the anchor items assessing perceived burden, limitations in daily activities and need for help into a dichotomous external criterion to estimate thresholds for clinical importance using Receiver Operator Characteristic (ROC) analysis.
Results: In our sample of 548 cancer patients (mean age 60.6 years; 54 % female), the QLQ-C30 scales showed high diagnostic accuracy in identifying patients reporting burden, limitations and/or need for help related to PF, EF, PA and FA. All areas under the curve were above 0.86.
Conclusions: We were able to estimate thresholds for clinical importance for four QLQ-C30 scales. When used in daily clinical practice, these thresholds can help to identify patients with clinically important problems requiring further exploration and possibly intervention by health care professionals.
Keywords: Cancer; EORTC QLQ-C30; Patient-reported outcomes; Quality of life; Threshold for clinical importance.
Figures
References
-
- Bottomley A, Biganzoli L, Cufer T, Coleman Robert E, Coens C, Efficace F, et al. Randomized, controlled trial investigating short-term health-related quality of life with doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in patients with metastatic breast cancer: European Organization for Research and Treatment of Cancer Breast Cancer Group, Investigational Drug Branch for Breast Cancer and the New Drug Development Group Study. J Clin Oncol. 2004;22:2576–86. doi: 10.1200/JCO.2004.02.037. - DOI - PubMed
-
- Bottomley A, Gaafar R, Gaafa R, Manegold C, Burgers S, Coens C, et al. Short-term treatment-related symptoms and quality of life: results from an international randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an EORTC Lung-Cancer Group and National Cancer Institute, Canada, Intergroup Study. J Clin Oncol. 2006;24:1435–42. doi: 10.1200/JCO.2005.03.3027. - DOI - PubMed
-
- van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–9. doi: 10.1200/JCO.20005.14.589. - DOI - PubMed
-
- Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480–501. doi: 10.1200/JCO.2013.53.5948. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical