Deficiency of cold-inducible ribonucleic acid-binding protein reduces renal injury after ischemia-reperfusion
- PMID: 27267546
- PMCID: PMC4938765
- DOI: 10.1016/j.surg.2016.04.014
Deficiency of cold-inducible ribonucleic acid-binding protein reduces renal injury after ischemia-reperfusion
Abstract
Background: Renal ischemia-reperfusion injury, commonly caused by major operation and shock, leads to acute kidney injury and is associated with high morbidity and mortality. Cold-inducible ribonucleic acid-binding protein, a cold shock protein, has recently been identified as a damage-associated molecular pattern. We hypothesized that cold-inducible ribonucleic acid-binding protein exacerbates severity of injury in renal ischemia-reperfusion.
Methods: Renal ischemia was induced in an 8-week-old male C57BL/6 wild-type mice and Cirp(-/-) mice via bilateral clamping of renal pedicles for 30 minutes, followed by reperfusion for 5 or 24 hours and harvest of blood and renal tissue for analysis. Anti-cold-inducible ribonucleic acid-binding protein antibody or non-immunized immunoglobulin G (IgG) was injected intravenously (10 mg/kg body weight) at time of reperfusion.
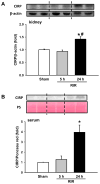
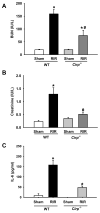
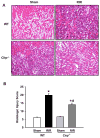
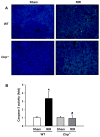
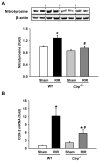
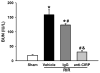
Results: After renal ischemia-reperfusion, Cirp(-/-) mice demonstrated a reduction of blood urea nitrogen and creatinine of 53% and 60%, respectively, compared with wild-type mice. Serum IL-6 levels were reduced significantly: 70% in Cirp(-/-) mice compared with wild-type mice after renal ischemia-reperfusion. Levels of nitrotyrosine, an oxidatively modified protein marker, and cyclooxygenase-2, an inflammatory mediator, also were significantly decreased in the kidneys of the Cirp(-/-) mice compared with wild-type mice after renal ischemia-reperfusion. Renal caspase-3 activity was decreased in Cirp(-/-) mice compared with wild-type mice after renal ischemia-reperfusion, which corresponded to the reduction of apoptotic cells determined by terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. Injection of neutralizing anti-cold-inducible ribonucleic acid-binding protein antibody into wild-type mice led to an 82% reduction in blood urea nitrogen compared with the vehicle after renal ischemia-reperfusion.
Conclusion: Deficiency of cold-inducible ribonucleic acid-binding protein results in less renal injury after renal ischemia-reperfusion by attenuating inflammation and oxidative stress. Furthermore, blockade of cold-inducible ribonucleic acid-binding protein shows a protective effect, indicating cold-inducible ribonucleic acid-binding protein as a target in the treatment of renal ischemia-reperfusion.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Tian H, Sun T, Hao D, Wang T, Li Z, Han S, et al. The optimal timing of continuous renal replacement therapy for patients with sepsis-induced acute kidney injury. Int Urol Nephrol. 2014;46:2009–14. - PubMed
-
- Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13:176–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

