Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow
- PMID: 27267759
- PMCID: PMC4897950
- DOI: 10.1186/s13041-016-0243-1
Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow
Abstract
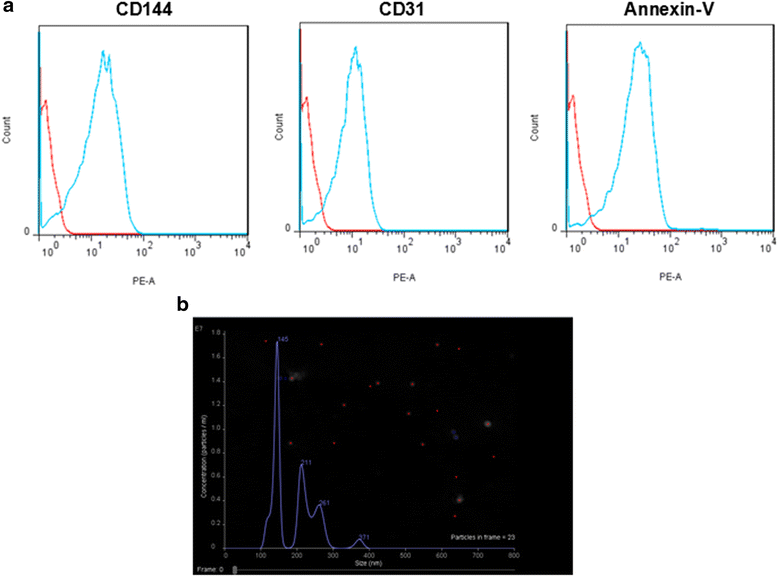
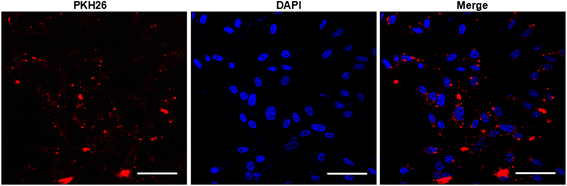
Background: Endothelial cell (EC) released microvesicles (EMVs) can affect various target cells by transferring carried genetic information. Astrocytes are the main components of the blood brain barrier (BBB) structure in the brain and participate in regulating BBB integrity and blood flow. The interactions between ECs and astrocytes are essential for BBB integrity in homeostasis and pathological conditions. Here, we studied the effects of human brain microvascular ECs released EMVs on astrocyte functions. Additionally, we investigated the effects of EMVs treated astrocytes on regulating BBB function and cerebral ischemic damage.
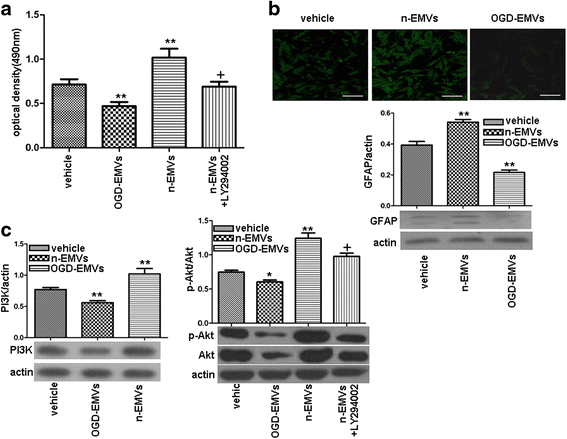
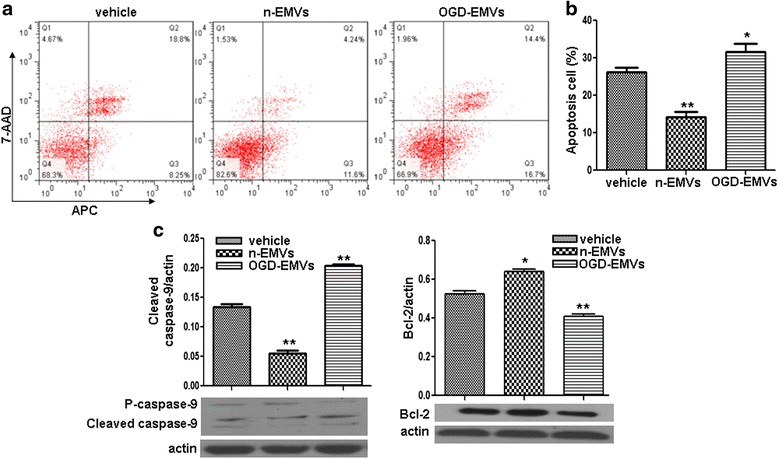
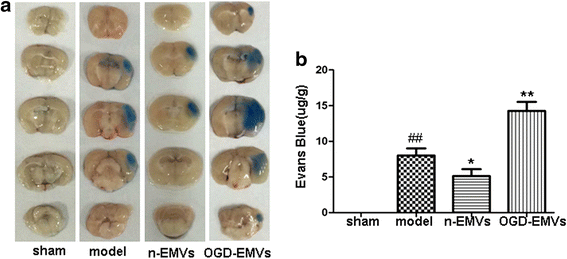
Results: EMVs prepared from ECs cultured in normal condition (n-EMVs) or oxygen and glucose deprivation (OGD-EMVs) condition had diverse effects on astrocytes. The n-EMVs promoted, while the OGD-EMVs inhibited the proliferation of astrocytes via regulating PI3K/Akt pathway. Glial fibrillary acidic protein (GFAP) expression (marker of astrocyte activation) was up-regulated by n-EMVs, while down-regulated by OGD-EMVs. Meanwhile, n-EMVs inhibited but OGD-EMVs promoted the apoptosis of astrocytes accompanied by up/down-regulating the expression of Caspase-9 and Bcl-2. In the BBB model of ECs-astrocytes co-culture, the n-EMVs, conversely to OGD-EMVs, decreased the permeability of BBB accompanied with up-regulation of zonula occudens-1(ZO-1) and Claudin-5. In a transient cerebral ischemia mouse model, n-EMVs ameliorated, while OGD-EMVs aggravated, BBB disruption, local cerebral blood flow (CBF) reduction, infarct volume and neurological deficit score.
Conclusions: Our data suggest that EMVs diversely modulate astrocyte functions, BBB integrity and CBF, and could serve as a novel therapeutic target for ischemic stroke.
Keywords: Astrocytes; Blood brain barrier; Cerebral blood flow; Cerebral ischemia; Endothelial cells; Gene expression; Microvesicles.
Figures



References
-
- Zhu H, Wang Z, Xing Y, Gao Y, Ma T, Lou L, et al. Baicalin reduces the permeability of the blood–brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. J Ethnopharmacol. 2012;141(2):714–20. doi: 10.1016/j.jep.2011.08.063. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

